
����������Ԫ��X��Y��Z��W��Q��ԭ��������������Xԭ�Ӻ��������������Ǵ�����2����Y�ķ�����YF3�����и�ԭ�Ӿ��ﵽ8�����ȶ��ṹ��Z��W�dz���������Z������������ͻ���ϣ�W�ļ�������ͬ���������Ӱ뾶��С�ģ�Q��p�ܼ�����һ��δ�ɶԵ��ӡ��Իش��������⣺
��1���Ƚϵ�һ�����ܣ�Z W�����������������������ͬ�����縺�ԣ�X Y��
��2��д��Q�ļ۵����Ų�ͼ ��YF3�Ľṹʽ ��
��3��������ͭ��Һ����ε���Y���⻯���ˮ��Һ�������������ӷ���ʽ��ʾ�ù��̳��ֵ�����仯��
_________________________ ��
��1������ ���� ��2�� ��
��  ��
��
��3��Cu2++2NH3��H2O = Cu(OH)2��+2NH4+��
Cu(OH)2+4NH3 = [Cu(NH3)4]2++2OH- ����Cu(OH)2+4NH3��H2O = [Cu(NH3)4]2++2OH-+4H2O��
�����������������������Ԫ��X��Y��Z��W��Q��ԭ��������������Xԭ�Ӻ��������������Ǵ�����2���������������������ܳ���8������Xԭ����2�����Ӳ㣬����������Ϊ4����XΪ̼Ԫ�أ�Z��W�dz���������ԭ����������̼Ԫ�أ�����ǵ�������Ԫ�ء�Z������������ͻ���ϣ�W�ļ�������ͬ���������Ӱ뾶��С�ģ���WΪAlԪ�ء�ZΪMgԪ�أ�Y�ķ�����YF3�����и�ԭ�Ӿ��ﵽ8�����ȶ��ṹ�����YΪ�ǽ�������Y����+3�ۣ�����������Ϊ8��3��5�����ڢ�A�壬ԭ������С��MgԪ�أ���YΪ��Ԫ�أ�Q���ڵ������ڣ�p�ܼ�����һ��δ�ɶԵ��ӣ���Χ�����Ų�Ϊ3s23p1��3s23p5������WΪAlԪ�أ���QΪClԪ�ء�
��1��������Խ������һ������Խ������Mgԭ��3s�ܼ�����2�����ӣ�Ϊȫ���ȶ�״̬�������ϵͣ���һ�����ܱ�AlԪ�صĸߣ�ͬ����������ҵ縺�������ʵ縺��C��N����
��2��QΪClԪ�أ���Χ�����Ų�Ϊ3s23p5��������Ԫ�صļ۵����Ų�ͼΪ ��NF3�е�ԭ�����ԭ��֮���γ�1�Թ��õ��Ӷԣ����ԽṹʽΪ��
��NF3�е�ԭ�����ԭ��֮���γ�1�Թ��õ��Ӷԣ����ԽṹʽΪ�� ��
��
��3��������ͭ��Һ����ε��백ˮ��Һ��������������������ͭ������泥�����ɫ�������ɡ������μӹ����İ�ˮ��������ͭ�백ˮ��Ӧ�����İ���ͭ�����ӣ���ɫ������ʧ����Һ������ɫ����Ӧ���ӷ���ʽΪ��Cu2++2NH3?H2O��Cu(OH)2��+2NH4+��Cu(OH)2+4NH3��H2O = [Cu(NH3)4]2++2OH-+4H2O��
���㣺����ṹ�����ʡ�λ�ù�ϵ��Ԫ�������ɡ���������Ų����������γɵ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1���������߷ֱ��ʾԪ�ص�ij��������˵�����Ĺ�ϵ(ZΪ�˵������YΪԪ�ص��й�����)����������Ԫ���йص�������������߱��������Ӧ�Ŀո��У�
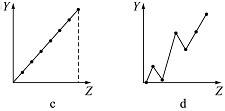
�٢�A��Ԫ�صļ۵�����__________��
�ڵ�������Ԫ�ص��������__________��
��F����Na����Mg2����Al3�������Ӱ뾶__________��
��2��Ԫ��X��Y��Z��M��N��Ϊ����������Ԫ�أ���ԭ����������������֪Yԭ������������������������֮��Ϊ3��4��MԪ��ԭ�ӵ���������������Ӳ���֮��Ϊ4��3��N����Z����X�����ӵİ뾶��С��������XN������Ϊ���塣�ݴ˻ش�
�ٻ�����XN�Ļ�ѧʽΪ________��
�ڻ�����A��B��Ϊ����������Ԫ���е���������Ԫ����ɵ�ǿ����ʣ�����������ˮ��Һ���ʼ��ԣ����Ԫ�ص�ԭ����Ŀ֮�Ⱦ�Ϊ1��1��1��B�Ǽ�ͥ��84������Һ����Ч�ɷݡ�����A�еĻ�ѧ������Ϊ__________��B�Ļ�ѧʽΪ ��
�۹�ҵ����ȡ����M�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
W��X��y��Z��ԭ���������������ͬһ������Ԫ�أ�W��X�ǽ���Ԫ�أ�Y��Z�Ƿǽ���Ԫ�ء�
��1��W��X���Ե�����������Ӧ��ˮ������Է�Ӧ�����κ�ˮ���÷�Ӧ�����ӷ���ʽΪ________________________________________________________________ ��
��2��W��Y���γɻ�����W2Y���û�����ĵ���ʽΪ________�������������Ũ�ȵ�W
������������Ӧ��ˮ�����Y���⻯���ϣ��仯ѧ����ʽΪ__________________��
��3��Y�ĵͼ�������ͨ��Z���ʵ�ˮ��Һ�У�������Ӧ�Ļ�ѧ����ʽΪ_____________________________________________________��
��4��W��X��Y��Z����Ԫ�ؼ����ӵ����Ӱ뾶��С�����˳����_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ֶ�����Ԫ�ص����ʻ�ṹ��Ϣ���±����������Ϣ�ش��������⡣
| Ԫ�� | T | X | Y | Z |
| ���� �ṹ ��Ϣ | �����ں�������Ԫ�أ����䵥���dz�������ȼ���� | ����Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷԣ������µ������������ȶ�������ԭ�ӽϻ��á� | ��������������ɫ���塢������ǿ�� �����ڿ�����ȼ�շ�����ɫ�Ļ��档 | ��������Ԫ�صļ������а뾶��С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E���Ƕ�����Ԫ�أ�ԭ��������������B��Cͬ���ڣ�A��Dͬ���塣A��C���γ�����Һ̬��������ң�ԭ�Ӹ����ȷֱ�Ϊ2��1��1��1��E �ǵؿ��к�����ߵĽ���Ԫ�ء�����������Ϣ�ش��������⣺
�żס����������к��зǼ��Թ��ۼ������ʵĵ���ʽ��_________��E���ӵĵ����Ų�ʽΪ____________��Cԭ�ӵĵ����Ų�ͼΪ ��Dԭ�ӵ�ԭ�ӽṹʾ��ͼΪ_______ ��
��2��B���⻯��ķе����ͬ����Ԫ���⻯��ķе㣬ԭ����_____________�����⻯��ĵ���ʽΪ_______������ԭ�ӵ��ӻ���ʽΪ _______�����ӵ����幹��Ϊ_______��
��3��DCA��E������������ˮ�������Ӧ�����ӷ���ʽ ��
��4�����ݶԽ��߹���Be��E�������ƣ�д��Be��DCA��Һ��Ӧ�����ӷ���ʽ______________________
��5����Ҫ˵���ǽ���Ԫ��X��Ԫ��Y��X��Y��Ϊ��ϡ������Ԫ�أ��ķǽ�����ǿ�������з�����ȷ����__________������ţ���
��X���ʿ���Y�����⻯�����û�����
��Xԭ�ӵ�������������Yԭ�ӵ�������������
��X���������ˮ��������Ա�Y���������ˮ���������ǿ
����H2����ʱX���ʱ�Y��������
�ݵ縺�ԣ�X>Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��X��Y��Z��W���ֺ�14�����ӵ����ӣ���ṹ�ص����£�
| ���Ӵ��� | X | Y | Z | W |
| ԭ�Ӻ��� | ���� | ��ͬԪ�ع��ɵ����� | ͬԪ�ع��ɵ����� | ͬԪ�ع��ɵ����� |
| ���ӵĵ���� | 0 | 0 | ��������� | 0 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���в��ֶ���������Ԫ�ص��й���Ϣ�����±���
| Ԫ�ر�� | T | X | Y | Z | W |
| Ԫ�ص����ʻ�ԭ�ӽṹ��� | ����������Ԫ����ԭ�Ӱ뾶��� | ������ϵĵ������ȴ������1���ҵ��������� | �����13���˶�״̬��ͬ�ĵ��� | ����������Һ̬����������е�ϵͿ��Ȼ�����ĵ��� | ������5�ֲ�ͬ�����ĵ���������������δ�ɶԵĵ��� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F��GԪ��ԭ����������������֪Bԭ���������3��δ�ɶԵ��ӣ�Cԭ������������������������֮��Ϊ3��4��E��Cͬ���壬F-��D+��A+���ӵİ뾶��С��������AF������Ϊ���壬G�Ļ�̬ԭ�Ӻ���M�ܲ��������ӣ�N�ܲ�ֻ��1�����ӡ�
�ݴ˻ش��������⣺
��1��д��DԪ�ػ�̬ԭ�ӵĺ�������Ų�ʽ ��B��C��E����Ԫ�صĵ�һ�������ɴ�С��˳����(��Ԫ�ط��ű�ʾ)
��2��A��C���γ�1 8���ӷ��ӣ���ˮ��Һ�е��������Ȼ�����Һʱ�д��������ݳ���д���÷�Ӧ�Ļ�ѧ����ʽ
��3��ij����������������Ԫ���е�����Ԫ����ɣ�Ϊ������������������Ҫ�ɷ֣����л�ѧ������Ϊ ���û�����ˮ��Һ�������Ե�ԭ����(�����ӷ���ʽ��ʾ) ��
��4��0.3molG�ĵͼ����������� molB������������Ӧˮ�������Һǡ����ȫ��Ӧ(�軹ԭ����ֻ��BO)��
��5����������ʱ����B2A4Ϊȼ�ϣ�l mol��̬B2A4������C2��ȼ�գ�����B2����̬A2C���ų�534 kJ��������l molҺ̬A2C��ȫ����������44 kJ��������д����̬B2A4��C2��ȼ������B2��Һ̬A2Cʱ���Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ԭ������������������ֶ�����Ԫ��A��B��C��D��E����֪ A��Eͬ���壬A������Ԫ����ԭ�ӽṹ���BԪ��ԭ�ӵ��������������ڲ��������2����CԪ�ص�����������ˮ����X�����⻯�ﷴӦ����һ����Y��A��B��C��E����Ԫ�ض�����DԪ�طֱ��γ�ԭ�Ӹ����Ȳ���ͬ�ij���������ش��������⣺
��1��B�����ڱ��е�λ���� ��E��ԭ�ӽṹʾ��ͼ ��
��2��A��C�γɻ������к��еĻ�ѧ�����ڣ�����Լ����Ǽ��Լ����� ��
��3���õ���ʽ��ʾ������E2D���γɹ��� ��
��4��CԪ�ص�����������ˮ���������⻯�ﷴӦ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com