
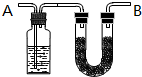
 ������C��H��O���л���3.24gװ��Ԫ�ط���װ�ã�ͨ��������O2ʹ֮��ȫȼ�գ������ɵ���������ͨ����A��B�����A������������2.16g��B��������9.24g����֪���л������Է�������Ϊ108��
������C��H��O���л���3.24gװ��Ԫ�ط���װ�ã�ͨ��������O2ʹ֮��ȫȼ�գ������ɵ���������ͨ����A��B�����A������������2.16g��B��������9.24g����֪���л������Է�������Ϊ108�� ��
�� ���� ��1������ͼʾ��֪Aװ����Һ��ӦΪ�����������ˮ��Bװ�����ն�����̼��
��2��Aװ������������2.16gΪ����ˮ��������B������������9.24gΪ���ɶ�����̼��������
����n=$\frac{m}{M}$�����л��ˮ��������̼�����ʵ���������ԭ���غ�����л��������N��C����N��H�����ٸ����л������Է����������������N��O�����ݴ˽��
��3������1������������л���ķ���ʽ��д���ܵĽṹ��
��� �⣺��1������ͼʾ��֪Aװ����Һ��ӦΪ�����������ˮ������Һ������ΪŨ���ᣬBװ�����ն�����̼��������̼Ϊ�������壬Ӧ�ü�ʯ�����գ�
�ʴ�Ϊ��Ũ�����ʯ�ң�
��2��A��ˮ��������2.16g��Ϊ����ˮ��������ˮ�����ʵ���Ϊ$\frac{2.16g}{18g/mol}$=0.12mol����H0.24mol��
��ʯ����CO2����9.24g�������ɶ�����̼�����ʵ���Ϊ$\frac{9.24g}{44g/mol}$=0.21mol��
���л������ʵ���Ϊ$\frac{3.24g}{108g/mol}$=0.03mol��
������������N��C��=$\frac{0.21mol}{0.03mol}$=7��
N��H��=$\frac{0.24mol}{0.03mol}$=8��
N��O��=$\frac{108-12��7-8}{16}$=1��
�����л���ķ���ʽΪC7H8O��
�ʴ�Ϊ��C7H8O��
��3�������л������ڷ����廯�������1��������C7H8O�IJ����Ͷ�=$\frac{2��7+2-8}{2}$=4���������������ͼ�����ֻ��1����������Ϊ-CH2OH��-OCH3����������2����Ϊ-OH��-CH3�����ڡ��䡢������λ�ù�ϵ���ʷ����������л���Ľṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л������ʽ�ļ��㣬��Ŀ�Ѷ��еȣ�����ע������л���ȼ�ղ����������ϵȷ���л���ķ���ʽ��Ϊ������Ĺؼ���ע�����л���������жϿ��ܵĽṹ��


 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ȫ�� | B�� | �٢ۢ� | C�� | �٢ۢܢ� | D�� | �ڢۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
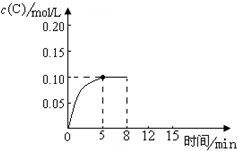 ��500��ʱ����������A����Ͷ��2.0L����ܱ������У�����A��s���T2B��g��+C��g����Ӧ���������C��ʱ���Ũ�ȱ仯��ͼ��ʾ
��500��ʱ����������A����Ͷ��2.0L����ܱ������У�����A��s���T2B��g��+C��g����Ӧ���������C��ʱ���Ũ�ȱ仯��ͼ��ʾ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ڢ� | C�� | ?�٢� | D�� | �ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | CO2���Ǵ�����Ⱦ����Կ��Դ����ŷŵ������� | |
| B�� | ������ϴ�Ӽ����溬��ϴ�Ӽ� | |
| C�� | ʵ�����ж��к�����Ӧ�����ռ���ͳһ���� | |
| D�� | ��ͨ�������ȡ����ʱ����NaOH��Һ����β�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���Ϳ��������ƻ�ױƷ | |
| B�� | �������ֿ�����ʳƷ���걾��ľ�ĵȵķ��� | |
| C�� | ʳƷ�����еIJ�������ֻ�ܴӲ�������ȡ������ͨ���л��ϳ����� | |
| D�� | ��Ұ�⣬�����������˺�ͿĨ�ռ�Һ���Ի�����ע��Ƥ���ڵ����������ʹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 2015������3.15�����ع���ɽ��ʡ��Ӫ�С���������������̣���һ����90�ŵ��������ϴ�����ʯ���͡�����������ȩ�ȵ��ͳɡ�93�����͡���������֪������ȩ��һ����ɫ�ӷ���ȼҺ�壬��Ҫ��������ɱ�����Ƥ��������Ϲ���ȣ������еij��������ұ��в������ӵ��к����ʣ��������������·©�ͣ��Զ������������ã�Ҳ��һ�����ԣ����ж���˵������ȷ���ǣ�������
2015������3.15�����ع���ɽ��ʡ��Ӫ�С���������������̣���һ����90�ŵ��������ϴ�����ʯ���͡�����������ȩ�ȵ��ͳɡ�93�����͡���������֪������ȩ��һ����ɫ�ӷ���ȼҺ�壬��Ҫ��������ɱ�����Ƥ��������Ϲ���ȣ������еij��������ұ��в������ӵ��к����ʣ��������������·©�ͣ��Զ������������ã�Ҳ��һ�����ԣ����ж���˵������ȷ���ǣ�������| A�� | ����ȩ����ȩ��Ϊͬϵ�� | |
| B�� | �ü״��ͼ�ȩ��������ȩ�ķ�ӦΪ�ӳɷ�Ӧ | |
| C�� | �����ʵ����ļ���ȩ�ͱ�Ȳ��ȫȼ�յĺ�������ͬ | |
| D�� | �ü���ȩ����ɱ�����Ƥ��������Ϲ���ȣ��������������Ķ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ڢ� | C�� | �ۢ� | D�� | �٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
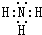 ��A�����ڱ��е�λ�õ�һ���ڵڢ�A�壮
��A�����ڱ��е�λ�õ�һ���ڵڢ�A�壮�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com