
������ͭˮ��Һ����μ��백ˮ�����γ���ɫ�����������μӰ�ˮ�������ܽ⣬�õ�����ɫ������Һ��������Һ�м����Ҵ�������ɫ���壨��ѧʽΪ[Cu(NH3)4]SO4��H2O��������
(1)д������ʵ��ǰ������Ӧ�����ӷ���ʽ__________��____________��
(2)ͭԪ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ_________________��ͭ���ʾ����е�ԭ��ѻ�ģ������_________�ѻ�����ѻ�ģ�����ƣ���
(3)����������ɫ���������ķǽ���Ԫ���У��縺��������_________����Ԫ�ط��ţ�����һ������������_________����Ԫ�ط��ţ����þ����е������ӵ����幹����_________�������ӵ�����ԭ�ӵ��ӻ���ʽΪ_________��
(4)���ķе�_________������ڡ����ڡ����(PH2)��ԭ����_____________��
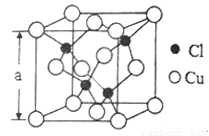
(5)Cu��һ���Ȼ��ᄃ���ṹ��ͼ��ʾ�����Ȼ���Ļ�ѧʽ��______________�����þ�����ܶ�Ϊpg��cm-3����NA��ʾ����٤����������þ����ı߳�Ϊa=_____________nm��
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ��ɳ��2016-2017ѧ���һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ѡ����
��ͭƬ����ʢ������ϡ������ձ��У���ʹͭƬ�ܽ⣬�������ձ��м���
A. ���Ȼ������� B. ����粒���
C. ϡ������Һ D. ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�츣��ʡ�����е���У�����ڶ������Ͽ������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
���ᣨH2C2O4���Ƕ�Ԫ���ᣨK1=5.9��10-2��K2=6.4��10-5������10mLϡH2C2O4��Һ�еμӵ�Ũ��NaOH��Һ��H2C2O4��HC2O4����C2O42����Ũ�ȷ���������ҺpH�仯�Ĺ�ϵ��ͼ������˵����ȷ����(����)
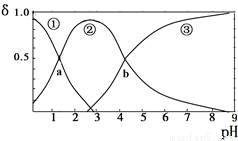
A. HC2O4����Ũ�ȷ�����pH���������
B. ����a����Ӧ�����NaOH��Һ�����Ϊ5mL
C. ����b��c(H+)=6.4��10-5
D. pH=5ʱ����c(Na+)+c(H+)��c(C2O42��)+c(HC2O4��)+c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�ϲ��и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����л����������ȷ����
A. (CH2CH2)2CHCH3 2-�һ�����
B. 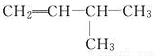 2-��-3-��ϩ
2-��-3-��ϩ
C. CH3C CCH3 2-��Ȳ
CCH3 2-��Ȳ
D. 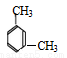 1��5-���ױ�
1��5-���ױ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�ϲ��и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ͨ����ѧ��Ӧʹ��ˮ��ɫ������ʹ���Ը��������Һ��ɫ����
A. �� B. ���� C. ����ϩ D. ��ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡ�����и߶���ѧ�ڵ�һ���¿������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
ֱ�Ӱ�����(NH3��BH3)��ؿ��ڳ����¹�����װ������ͼ���õ�ص��ܷ�ӦΪNH3��BH3+3H2O2=NH4BO2+4H2O������˵����ȷ���ǣ� ��
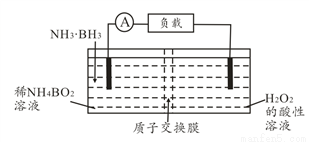
A. ���缫������ԭ��Ӧ
B. ��ع���ʱ��H+ͨ�����ӽ���Ĥ���ƶ�
C. �����ĵ缫��ӦʽΪ2H++2e��=H2��
D. ����3.1g�����飬������ת��0.6mol����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ���ٴ�һ�и���1���������ۻ�ѧ�Ծ��������棩 ���ͣ������
��֪ͭ�������A���ṹ��ͼ������ش��������⣺



(1)Cu�ļ����Ų�ʽΪ______________ ��
(2)A��������Ԫ��C��N��O�ĵ�һ�������ɴ�С��˳��Ϊ_______________�����е�ԭ�ӵ��ӻ��������Ϊ________��
(3)���就�������(H2NCH2COO-)���ȷֽ�ɲ���CO2��N2��N2�ЦҼ��ͦм���Ŀ֮��
��__________��N2O��CO2��Ϊ�ȵ����壬��N2O������Oֻ��һ��N��������N2O�ĵ���ʽΪ_______��
(4)��Cu���£��״��ɱ�����Ϊ��ȩ(HCHO)����ȩ������H CO�ļ���___________��ѡ����ڡ��������ڡ���С�ڡ���120�㣻��ȩ����ˮ�γ������������ͼ�б�ʾ����___________��
(5)������������ͼ������ʯ�ṹ���ƣ��dz�Ӳ���ϡ���������������B-N��������ԭ����֮��Ϊ___________���ṹ��ѧ����ԭ�����������ʾ�����ڲ���ԭ�ӵ����λ�ã���ͼ���ң�����������ľ����У�Bԭ�ӵ���������ֱ��У�
 �ȡ��������������Bԭ������ҵȾ��Nԭ�ӵ��������Ϊ____________________ ��
�ȡ��������������Bԭ������ҵȾ��Nԭ�ӵ��������Ϊ____________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ����ˮ����ѧ������У����2��������ѧ�Ծ��������棩 ���ͣ�ѡ����
һ���¶��£������������Ϊ0.5 L�ĺ����ܱ������з�����Ӧ��
CO(g)+Cl2(g) COCl2(g)
COCl2(g)
������������5 minʱ����ƽ�⡣
������� | �¶�/�� | ��ʼ���ʵ���/mol | ƽ�����ʵ���/mol | ||
CO | Cl2 | COCl2 | COCl2 | ||
�� | 500 | 1.0 | 1.0 | 0 | 0.8 |
�� | 500 | 1.0 | a | 0 | 0.5 |
�� | 600 | 0.5 | 0.5 | 0.5 | 0.7 |
����˵����ȷ����
A. ��������ǰ5 min��ƽ����Ӧ����v(CO)=0.16 mol��L-1��min-1
B. �÷�Ӧ������ӦΪ���ȷ�Ӧ
C. ����������ʼʱCl2�����ʵ���Ϊ0.55 mol
D. ����ʼʱ���������м���CO 0.8 mol��Cl2 0.8 mol���ﵽƽ��ʱCO��ת���ʴ���80%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017���㽭ʡ̨���и�����ѧ����ĩ�����������Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵������ȷ����
A. ��������ʯӢ����Ҫ�ɷ�
B. �ƼغϽ�����ڿ����ӷ�Ӧ�ѵ��Ƚ�����
C. С�մ�����ڱ��Ƹ��
D. ˮ������¶�ڿ����лᷢ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com