
ijŹµŃ銔×éĄūÓĆ·“Ó¦2CuO£«2Cl2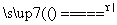 2CuCl2£«O2²ā¶ØĶµÄ½üĖĘĻą¶ŌŌ×ÓÖŹĮ棬æɹ©Ń”ŌńµÄ×°ÖĆČēĶ¼ĖłŹ¾”£
2CuCl2£«O2²ā¶ØĶµÄ½üĖĘĻą¶ŌŌ×ÓÖŹĮ棬æɹ©Ń”ŌńµÄ×°ÖĆČēĶ¼ĖłŹ¾”£
·½°øŅ»£ŗĶعż²ā¶Ø·“Ó¦ĪļCuOµÄÖŹĮæm(CuO)ŗĶ²śĪļO2µÄĢå»żV(O2)Ą“²ā¶ØĶµÄ½üĖĘĻą¶ŌŌ×ÓÖŹĮ攣
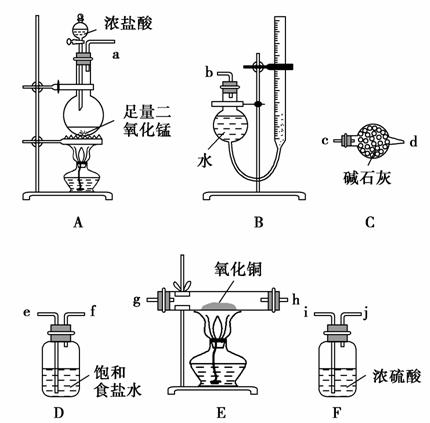
(1)°“ĘųĮ÷·½Ļņ“Ó×óµ½ÓŅÓĆ½ŗ¹Ü(Ķ¼ÖŠĪ“»³ö)½«Ń”ŌńµÄŅĒĘ÷×éŗĻ³ÉŅ»Ģ׏µŃé×°ÖĆ£¬Į¬½ÓĖ³ŠņĪŖa”ś (””””) (””””)”ś (””””) (””””)”ś (””””) (””””)”ś (””””) (””””)”śb”£
(2)×°ÖĆBŹĒÓÉøÉŌļ¹ÜŗĶ¼īŹ½µĪ¶Ø¹ÜøÄŌģ¶ų³ÉµÄ²āĮæĘųĢåĢå»żµÄ×°ÖĆ£¬ŹµŃéĒ°µĪ¶Ø¹ÜŅŗĆę³õ¶ĮŹżĪŖV1 L£¬ŹµŃéŗó»Öø“µ½ŹŅĪĀ£¬µ÷½Ś×°ÖĆĮ½²ąŅŗĆęĻąĘ½ŗóµĆµ½Ä©¶ĮŹżĪŖV2 L£¬ÉčŹŅĪĀŹ±ĘųĢåĦ¶ūĢå»żĪŖVm L”¤mol£1£¬ĒŅE×°ÖĆÖŠCuOµÄÖŹĮæĪŖm1 g£¬³ä·Ö·“Ó¦ŗóÉś³ÉCuCl2µÄÖŹĮæĪŖm2 g£¬ŌņĶµÄ½üĖĘĻą¶ŌŌ×ÓÖŹĮæµÄ±ķ“ļŹ½ĪŖ______________________________________________________ [ÓĆŗ¬m1”¢V1”¢V2µÄ“śŹżŹ½±ķŹ¾]”£
(3)ČōŃõ»ÆĶÖŠ»ģÓŠĶ£¬Ōņ²ā¶Ø½į¹ū________(Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°ĪŽÓ°Ļģ”±)”£
(4)×°ÖĆEŌŚŹµŃé¹ż³ĢÖŠµÄÖ÷ŅŖĻÖĻóŹĒ__________________________________ _________________________________________________________________”£
·½°ø¶ž£ŗĄūÓĆA”¢D”¢E”¢FĖÄĢ××°ÖĆ(Ī²ĘųÓÉĘäĖūµÄ×°ÖĆ“¦Ąķ)Ķź³É²ā¶ØČĪĪń”£
(5)ÄćČĻĪŖ²ā¶ØµÄĪļĄķĮæÓŠ___________________________________________
(Š“³öŅ»×é)£¬°“Äć²ā¶ØµÄĪļĄķĮ棬Š“³öĶµÄ½üĖĘĻą¶ŌŌ×ÓÖŹĮæµÄ±ķ“ļŹ½£ŗ__________________________________________________________________
_________________________________________________________________ӣ
½āĪö””·½°øŅ»£ŗĶعż²ā¶Ø·“Ó¦ĪļCuOµÄÖŹĮæm(CuO)ŗĶ²śĪļO2µÄĢå»żV(O2)Ą“²ā¶ØĶµÄ½üĖĘĻą¶ŌŌ×ÓÖŹĮ棻·½°ø¶ž£ŗĶعż²ā¶Ø·“Ó¦ĪļCuOµÄÖŹĮæŗĶ²śĪļCuCl2µÄÖŹĮæĄ“²ā¶ØĶµÄ½üĖĘĻą¶ŌŌ×ÓÖŹĮ攣ŹµŃéÖŠŠčŅŖµĆµ½“æ¾»µÄCl2£¬×°ÖĆA£ŗMnO2£«4HCl(ÅØ)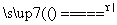 MnCl2£«Cl2”ü£«2H2O£¬µĆµ½Cl2ÖŠŗ¬ÓŠHClŗĶĖ®ÕōĘų£¬ÓĆ×°ÖĆDĄ“³żČ„HCl£¬ÓĆ×°ÖĆFøÉŌļCl2”£ŌŚ×°ÖĆEÖŠ·¢Éś·“Ó¦2CuO£«2Cl2
MnCl2£«Cl2”ü£«2H2O£¬µĆµ½Cl2ÖŠŗ¬ÓŠHClŗĶĖ®ÕōĘų£¬ÓĆ×°ÖĆDĄ“³żČ„HCl£¬ÓĆ×°ÖĆFøÉŌļCl2”£ŌŚ×°ÖĆEÖŠ·¢Éś·“Ó¦2CuO£«2Cl2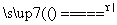 2CuCl2£«O2”£µ±ÓĆ·½°øŅ»Ź±£¬“ÓEÖŠ³öĄ“µÄĘųĢåŅŖĻČÓĆ×°ÖĆC(¼īŹÆ»Ņ)³żČ„¶ąÓąµÄĀČĘų£¬ŌŁÓĆ×°ÖĆB²āĮæV(O2)£¬Ņņ“Ė·½°øŅ»ŅĒĘ÷Į¬½ÓĖ³ŠņĪŖa”śef”śij”śgh”ścd”śb”£
2CuCl2£«O2”£µ±ÓĆ·½°øŅ»Ź±£¬“ÓEÖŠ³öĄ“µÄĘųĢåŅŖĻČÓĆ×°ÖĆC(¼īŹÆ»Ņ)³żČ„¶ąÓąµÄĀČĘų£¬ŌŁÓĆ×°ÖĆB²āĮæV(O2)£¬Ņņ“Ė·½°øŅ»ŅĒĘ÷Į¬½ÓĖ³ŠņĪŖa”śef”śij”śgh”ścd”śb”£
2CuO”””””””””””«””””””””””O2
2Ar(Cu)£«32 Vm
m1 V1£V2
 £½
£½ £¬M(Cu)£½
£¬M(Cu)£½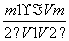 £16”£
£16”£
CuO”””””””””””””«””””””””””CuCl2
Ar(Cu)£«16 Ar(Cu)£«71
m1 m2
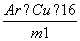 £½
£½ £¬
£¬
æɵĆAr(Cu)£½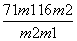 ”£
ӣ
“š°ø””(1)e””f””i””j””g””h””c””d
(2)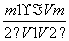 £16””(3)Ę«“ó
£16””(3)Ę«“ó
(4)¹ĢĢåÓÉŗŚÉ«±ä³É×Ų»ĘÉ«
(5)m1”¢m2””Ar(Cu)£½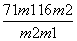


 Š”ѧɜ10·ÖÖÓæŚĖć²āŹŌ100·ÖĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓæŚĖć²āŹŌ100·ÖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»ÆѧÓėÉś²ś”¢Éś»ī”¢»·¾³ĆÜĒŠĻą¹Ų£®ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ£Ø£©
A£® ŗ½Ģģ·É»śÉĻµÄøōČČĢÕ“ÉĶߏōÓŚø“ŗĻ²ÄĮĻ
B£® ĮņĖįÄĘČÜŅŗŗĶĀČ»Æ±µČÜŅŗ¾łÄÜŹ¹µ°°×ÖŹ±äŠŌ
C£® ”°¹ā»ÆѧŃĢĪķ”±”¢”°ĻõĖįŠĶĖįÓź”±µÄŠĪ³É¶¼ÓėµŖŃõ»ÆĪļÓŠ¹Ų
D£® ĀĢÉ«»ÆѧµÄŗĖŠÄŹĒĄūÓĆ»ÆѧŌĄķ“ÓŌ“Ķ·ÉĻ¼õÉŁ»ņĻū³ż¹¤ŅµÉś²ś¶Ō»·¾³µÄĪŪČ¾
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»ÆŗĻĪļA£ØC4H10O£©ŹĒŅ»ÖÖÓŠ»śČܼĮ£®AæÉŅŌ·¢ÉśČēĻĀ±ä»Æ£ŗ
£Ø1£©A·Ö×Ó ÖŠµÄ¹ŁÄÜĶÅĆū³ĘŹĒ £»
ÖŠµÄ¹ŁÄÜĶÅĆū³ĘŹĒ £»
£Ø2£©AÖ»ÓŠŅ»ÖÖŅ»ĀČČ”“śĪļB£¬Š“³öÓÉA×Ŗ»ÆĪŖBµÄ»Æѧ·½³ĢŹ½£ŗ
£Ø3£©AµÄĶ¬·ÖŅģ¹¹ĢåFŅ²æÉŅŌ·¢ÉśæņĶ¼ÄŚAµÄø÷ÖÖ±ä»Æ£¬ĒŅFµÄŅ»ĀČČ”“śĪļÓŠČżÖÖ£¬Š“³öFµÄ½į¹¹¼ņŹ½ £®

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŠšŹö²»ÕżČ·µÄŹĒ (””””)”£
A£®CO2µÄĦ¶ūÖŹĮæŹĒ44 g”¤mol£1£¬±ķŹ¾ 1 mol CO2µÄÖŹĮæĪŖ44 g
B£®H2SO4ČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ 1 mol”¤L£1£¬±ķŹ¾ 1 LČÜŅŗÖŠŗ¬ÓŠ 1 mol H2SO4
C£®ĘųĢåĦ¶ūĢå»żVm”Ö22.4 L”¤mol£1£¬±ķŹ¾1 molČĪŗĪĘųĢåµÄĢå»ż¶¼Ō¼ĪŖ22.4 L
D£®°¢·ü¼ÓµĀĀŽ³£ŹżNA”Ö6.02”Į1023 mol£1£¬±ķŹ¾1 molČĪŗĪĮ£×Ó¼ÆĢåĖłŗ¬µÄĮ£×ÓŹżŌ¼ĪŖ6.02”Į1023
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓĆNA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£ŹżµÄÖµ”£ĻĀĮŠŠšŹöÖŠÕżČ·µÄŹĒ
(””””)”£
A£®NAøöN2·Ö×ÓÓė0.5NAøöH2·Ö×ÓĖłÕ¼µÄĢå»ż±ČŅ»¶ØŹĒ2”Ć1
B£®ĻąĶ¬ĪļÖŹµÄĮæµÄOH£ŗĶCH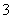 ŗ¬ÓŠĻąĶ¬ŹżÄæµÄµē×Ó
ŗ¬ÓŠĻąĶ¬ŹżÄæµÄµē×Ó
C£®25 ”ꏱ£¬pH£½13µÄBa(OH)2ČÜŅŗÖŠŗ¬ÓŠOH£µÄŹżÄæĪŖ0.2NA
D£®³£ĪĀ³£Ń¹ĻĀ£¬NO2Óė×ćĮæH2O·“Ӧɜ³É0.1 mol NO£¬Ōņ×ŖŅʵĵē×ÓŹżĪŖ0.2NA
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĀĮ¹čŗĻ½š(ŗ¬¹č15.5%)ŌŚÄż¹ĢŹ±ŹÕĖõĀŹŗÜŠ”£¬Ņņ¶ųÕāÖÖŗĻ½šŹŹŗĻÖżŌģ£¬ĻÖÓŠĻĀĮŠ3ÖÖ¾§Ģå£ŗ¢ŁĀĮ¢Ś¹č¢ŪĀĮ¹čŗĻ½š£¬ĖüĆĒµÄČŪµć“ÓµĶµ½øßµÄĖ³ŠņŹĒ (””””)”£
A£®¢Ł¢Ś¢Ū B£®¢Ś¢Ł¢Ū C£®¢Ū¢Ś¢Ł D£®¢Ū¢Ł¢Ś
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
³£¼ūµÄÓĄ¾Ć“ÅĢśµÄ×é³ÉĪŖBaFe12O19ÓėŌųÓĆÓŚĄ×“ļĪ¢²Ø¹żĀĖĘ÷ĄļµÄīĘĢśĮńŹÆ(Y3Fe5O12)¾łĪŖĢśŃõĢ唣īĘ(Y)ŹĒĻ”ĶĮŌŖĖŲ£¬³£ĪŖ£«3¼Ū”£ĖüĆĒµÄ×é³ÉÖŠĖłŗ¬ĢśŌŖĖŲµÄ¼ŪĢ¬Ó¦ĪŖ (””””)”£
A£®Ö»ÓŠ£«2¼Ū B£®Ö»ÓŠ£«3¼Ū
C£®£«2ŗĶ£«3¼Ū D£®£«2ŗĶ£«6¼Ū
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĢśŌŖĖŲŹĒČĖĢåµÄÖŲŅŖÓŖŃųŌŖĖŲ£¬ĢśŌŚČĖĢåÄŚµÄÖ÷ŅŖ¹¦ÄÜŹĒŅŌŃŖŗģµ°°×(ŗ¬µĶ¼ŪĢś)µÄŠĪŹ½²Ī¼ÓŃõµÄ×ŖŌĖ”¢½»»»ŗĶ×éÖÆŗōĪü¹ż³Ģ£¬ŅŌ¼°ŌŚĢåÄŚÓĆÓŚÉś²ś¹©øųÉśĆü»ī¶ÆŠčŅŖµÄĻø°ūÄÜĮæATP”£
(1)ČĖĢåÕż³£µÄŃŖŗģµ°°×Ó¦øĆŗ¬Fe2£«”£ČōĪóŹ³ŃĒĻõĖįŃĪ(ČēNaNO2)£¬Ōņµ¼ÖĀŃŖ
ŗģµ°°×ÖŠFe2£«×Ŗ»Æ³ÉFe3£«¶ųÖŠ¶¾£¬·žÓĆĪ¬ÉśĖŲCæɽā¶¾”£ĻĀĮŠŠšŹöÖŠÕżČ·µÄ
ŹĒ (””””)”£
A£®ŃĒĻõĖįŃĪŹĒ»¹Ō¼Į
B£®Ī¬ÉśĖŲCŹĒ»¹Ō¼Į
C£®Ī¬ÉśĖŲC½«Fe3£«»¹ŌĪŖFe2£«
D£®ŃĒĻõĖįŃĪ±»Ńõ»Æ
(2)ČéĖįæÉŅŌÓė¾«ÖĘĢś·ŪÖʱøŅ»ÖÖŅ©Īļ£¬·“Ó¦Ź½ĪŖ£ŗ
2CH3CH(OH)COOH£«Fe ===[CH3CH(OH)COO]2Fe£«H2”üŌŚøĆ·“Ó¦ÖŠ£¬Ńõ»Æ
¼ĮŹĒ________£¬»¹Ō¼ĮŹĒ________£¬²śĪļČéĖįŃĒĢśæÉŅŌÖĪĮĘµÄ¼²²”ŹĒ
____________ӣ
(3)¾ŁŅ»ĄżĖµĆ÷ĢśŌŖĖŲŌŚČĖĢåÖŠµÄÖŲŅŖ×÷ÓĆ____________________________
_________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŌĻĀ±ķŹ¾ŗ¤Ō×Ó½į¹¹µÄ»ÆѧÓĆÓļÖŠ£¬¶Ōµē×ÓŌĖ¶ÆדĢ¬ĆčŹö×īĻź¾”µÄŹĒ£Ø£©
A£® He B£®  C£® 1s2 D£®
C£® 1s2 D£® 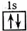
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com