
Β―ι¥Έ ΐ | Τπ ΦΈ¬Ε»t1/Γφ | ÷ΙΈ¬Ε»t2/Γφ | |
―ΈΥα | NaOH»ή“Κ÷’ | ||
1 | 20.2 | 20.3 | 23.7 |
2 | 20.3 | 20.5 | 23.8 |
3 | 21.5 | 21.6 | 24.9 |
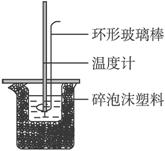
‘Άξ≥…œ¬Ν–Έ ΧβΘΚ
Θ®1Θ© Β―ι ±”ΟΜΖ–Έ≤ΘΝßΑτΫΝΑη»ή“ΚΒΡΖΫΖ® «________________________Θ§≤ΜΡή”ΟΆ≠ΥΩΫΝΑηΑτ¥ζΧφΜΖ–Έ≤ΘΝßΑτΒΡάμ”… «________________________________ΓΘ
Θ®2Θ©Ψ≠ ΐΨί¥ΠάμΘ§t2-t1=3.4 ΓφΓΘ‘ρΗΟ Β―ι≤βΒΟΒΡ÷–ΚΆ»»ΠΛH=________________Γ≤―ΈΥαΚΆNaOH»ή“ΚΒΡΟήΕ»Α¥1 gΓΛcm-3ΦΤΥψΘ§Ζ¥”ΠΚσΜλΚœ»ή“ΚΒΡ±»»»»ίΘ®cΑ¥4.18 JΓΛ(gΓΛΓφ)-1ΦΤΥψΘ©Γ≥ΓΘ
Θ®3Θ©»τΫΪNaOH»ή“ΚΗΡΈΣœύΆ§ΧεΜΐΓΔœύΆ§≈®Ε»ΒΡΑ±Υ°Θ§≤βΒΟ÷–ΚΆ»»ΈΣΠΛH1Θ§‘ρΠΛH1”κΠΛHΒΡΙΊœΒΈΣΘΚΠΛH1_____ΠΛHΘ®ΧνΓΑΘΦΓ±ΓΑΘΨΓ±ΜρΓΑ=Γ±Θ©Θ§άμ”… «_______________________ΓΘ
Θ®1Θ©…œœ¬ΫΝΕ·Θ®Μρ«α«αΫΝΕ·Θ© Ά≠¥Ϊ»»ΩλΘ§Ζά÷Ι»»ΝΩΥπ ß
Θ®2Θ©-56.8 kJΓΛmol-1
Θ®3Θ©ΘΨ NH3ΓΛH2OΒγάκΈϋ»»
ΫβΈωΘΚ±ΨΧβΩΦ≤ι÷–ΚΆ»»ΒΡ≤βΕ® Β―ιΘ§÷ς“Σ¥”≤ΌΉςΉΔ“β ¬œνΚΆ»θΒγΫβ÷ ΒΡ÷–ΚΆ»»Ϋ«Ε»ΩΦ¬«Θ§“ρ”ΟΒΡ «ΜΖ–Έ≤ΘΝßΑτΘ§÷ΜΡή…œœ¬¬ΐ¬ΐΫΝΑηΘ§ΩΦ¬«ΒΫΫπ τΒΡΒΦ»»–‘Θ§Ζά÷Ι»»ΝΩ…Δ ß≤Μ”ΟΆ≠ΥΩΘΜΑ±Υ° «»θΒγΫβ÷ Θ§÷–ΚΆΙΐ≥Χ÷–ΒγάκΈϋ»»Θ§÷–ΚΆ»» ΐ÷Β–ΓΓΘ


 ΩΦ«Α±ΊΝΖœΒΝ–¥πΑΗ
ΩΦ«Α±ΊΝΖœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
(1)≈δΤΫ…œ ωΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ_____________________________ΓΘ
(2)≈®―ΈΥα‘ΎΖ¥”Π÷–œ‘ Ψ≥ωά¥ΒΡ–‘÷ «_____________________(Χν–¥±ύΚ≈Θ§Εύ―ΓΒΙΩέΖ÷)ΓΘ
ΔΌ÷Μ”–ΜΙ‘≠–‘
ΔΎΜΙ‘≠–‘ΚΆΥα–‘
Δέ÷Μ”–―θΜ·–‘
Δή―θΜ·–‘ΚΆΥα–‘
(3)»τ≤ζ…ζ0.1 mol Cl2Θ§‘ρΉΣ“ΤΒγΉ”ΒΡΈο÷ ΒΡΝΩΈΣ __________molΓΘ
(4)»τΖ¥”Π÷–HClΒΡάϊ”Ο¬ ÷Μ”–50%Θ§Β±―θΜ·≤ζΈο±»ΜΙ‘≠≤ζΈοΕύ7.1 g ±Θ§«σ≈®―ΈΥαΒΡΈο÷ ΒΡΝΩ≈®Ε»ΘΩ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
Β―ι¥Έ ΐ | Τπ ΦΈ¬Ε»t1/Γφ | ÷’÷ΙΈ¬Ε»t2/Γφ | |
―ΈΥα | NaOH»ή“Κ | ||
1 | 20.2 | 20.3 | 23.7 |
2 | 20.3 | 20.5 | 23.8 |
3 | 21.5 | 21.6 | 24.9 |
‘ΜΊ¥πœ¬Ν–Έ ΧβΓΘ
(1) Β―ι ±”ΟΜΖ–Έ≤ΘΝßΑτΫΝΑη»ή“ΚΒΡΖΫΖ® «___________Θ§≤ΜΡή”ΟΆ≠ΥΩΫΝΑηΑτ¥ζΧφΜΖ–Έ≤ΘΝßΑτΒΡάμ”… «____________________________________________________________________ΓΘ

(2)Ψ≠ ΐΨί¥ΠάμΘ§t2-t1=3.4 ΓφΓΘ‘ρΗΟ Β―ι≤βΒΟΒΡ÷–ΚΆ»»ΠΛH=_______Γ≤―ΈΥαΚΆNaOH»ή“ΚΒΡΟήΕ»Α¥1 gΓΛcm-3ΦΤΥψΘ§Ζ¥”ΠΚσΜλΚœ»ή“ΚΒΡ±»»»»ί(cΑ¥4.18 JΓΛg-1ΓΛΓφ-1ΦΤΥψ)Γ≥ΓΘ
(3)»τΫΪNaOH»ή“ΚΗΡΈΣœύΆ§ΧεΜΐΓΔœύΆ§≈®Ε»ΒΡΑ±Υ°Θ§≤βΒΟ÷–ΚΆ»»ΈΣΠΛH1,‘ρΠΛH1”κΠΛHΒΡΙΊœΒΈΣΘΚΠΛH1_______ΠΛH(ΧνΓΑΘΦΓ±ΓΑΘΨΓ±ΜρΓΑ=Γ±)Θ§άμ”… « ________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
Β―ι “”Ο50 mL≈®―ΈΥαΗζΉψΝΩΒΡ¬»ΥαΦΊΙΧΧεΙ≤»»÷Τ»Γ¬»ΤχΘ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ(Έ¥≈δΤΫ)ΘΚKClO3+HCl![]() KCl+Cl2Γϋ+H2O
KCl+Cl2Γϋ+H2O
(1)≈δΤΫ…œ ωΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ_____________________________ΓΘ
(2)≈®―ΈΥα‘ΎΖ¥”Π÷–œ‘ Ψ≥ωά¥ΒΡ–‘÷ «_____________________ΓΘ
(3)»τ≤ζ…ζ6.72L Cl2Θ®±ξΉΦΉ¥ΩωΘ©Θ§‘ρΉΣ“ΤΒγΉ”ΒΡΈο÷ ΒΡΝΩΈΣ __________molΓΘ
(4)»τΖ¥”Π÷–HClΒΡάϊ”Ο¬ ÷Μ”–50%Θ§Β±―θΜ·≤ζΈο±»ΜΙ‘≠≤ζΈοΕύ7.1 g ±Θ§«σ≈®―ΈΥαΒΡΈο÷ ΒΡΝΩ≈®Ε»ΘΩ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014Ϋλ‘ΤΡœ ΓάΞΟς –ΗΏΕΰ9‘¬‘¬ΩΦΜ·―ß ‘ΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ Β―ιΧβ
(Ι≤6Ζ÷) Β―ι “”Ο50 mL 0.50 molΓΛLΘ≠1―ΈΥαΓΔ50 mL 0.55 molΓΛLΘ≠1 NaOH»ή“ΚΚΆ»γΆΦΥυ ΨΉΑ÷ΟΘ§Ϋχ––≤βΕ®÷–ΚΆ»»ΒΡ Β―ιΘ§ΒΟΒΫ±μ÷–ΒΡ ΐΨίΘΚ

|
Β―ι¥Έ ΐ |
Τπ ΦΈ¬Ε»t1/Γφ |
÷’÷ΙΈ¬Ε»t2/Γφ |
|
|
―ΈΥα |
NaOH»ή“Κ |
||
|
1 |
20.2 |
20.3 |
23.7 |
|
2 |
20.3 |
20.5 |
23.8 |
|
3 |
21.5 |
21.6 |
24.9 |
Άξ≥…œ¬Ν–Έ ΧβΘΚ
(1) Β―ι ±≤ΜΡή”ΟΆ≠ΥΩΫΝΑηΑτ¥ζΧφΜΖ–Έ≤ΘΝßΫΝΑηΑτΒΡάμ”… « ΓΘ
(2)‘Ύ≤ΌΉς’ΐ»ΖΒΡ«ΑΧαœ¬Θ§ΧαΗΏ÷–ΚΆ»»≤βΕ®ΉΦ»Ζ–‘ΒΡΙΊΦϋ « ΓΘ
(3)ΗυΨί…œ±μ÷–Υυ≤β ΐΨίΫχ––ΦΤΥψΘ§‘ρΗΟ Β―ι≤βΒΟΒΡ÷–ΚΆ»»ΠΛHΘΫ [―ΈΥαΚΆNaOH»ή“ΚΒΡΟήΕ»Α¥1 gΓΛcmΘ≠3ΦΤΥψΘ§Ζ¥”ΠΚσΜλΚœ»ή“ΚΒΡ±»»»»ί(c)Α¥4.18 JΓΛ(gΓΛΓφ)Θ≠1ΦΤΥψ]ΓΘ»γ”Ο0.5 mol/LΒΡ―ΈΥα”κNaOHΙΧΧεΫχ–– Β―ιΘ§‘ρ Β―ι÷–≤βΒΟΒΡΓΑ÷–ΚΆ»»Γ± ΐ÷ΒΫΪ (ΧνΓΑΤΪ¥σΓ±ΓΔΓΑΤΪ–ΓΓ±ΓΔ ΓΑ≤Μ±δΓ±)ΓΘ
Θ®4Θ©»τΡ≥Ά§―ßάϊ”Ο…œ ωΉΑ÷ΟΉω Β―ιΘ§”––©≤ΌΉς≤ΜΙφΖΕΘ§‘λ≥…≤βΒΟ÷–ΚΆ»»ΒΡ ΐ÷ΒΤΪΒΆΘ§«κΡψΖ÷ΈωΩ…ΡήΒΡ‘≠“ρ «
A.≤βΝΩ―ΈΥαΒΡΈ¬Ε»ΚσΘ§Έ¬Ε»ΦΤΟΜ”–”ΟΥ°≥εœ¥Η…ΨΜ
B.Α―ΝΩΆ≤÷–ΒΡ«β―θΜ·ΡΤ»ή“ΚΒΙ»κ–Γ…’±≠ ±Ε·Ής≥ΌΜΚ
C.Ήω±Ψ Β―ιΒΡΒ±Χλ “Έ¬ΫœΗΏ
D.ΫΪ50mL0.55mol/L«β―θΜ·ΡΤ»ή“Κ»Γ≥…ΝΥ50mL0.55mol/LΒΡΑ±Υ°
E.‘ΎΝΩ»Γ―ΈΥα ±―ω ”ΦΤ ΐ
F.¥σ…’±≠ΒΡΗ«Αε÷–Φδ–ΓΩΉΧΪ¥σ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2011-2012ΡξΚτ¬Ή±¥Εϊ –ΗΏ“Μœ¬―ßΤΎΤΎΡ©ΩΦ ‘Μ·―ß ‘ΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ Β―ιΧβ
Θ®6Ζ÷Θ© Β―ι “”Ο50 mL 0.50 molΓΛLΘ≠1―ΈΥαΓΔ50 mL 0.55 molΓΛLΘ≠1 NaOHΘ§άϊ”Ο»γΆΦΉΑ÷ΟΫχ––÷–ΚΆ»»ΒΡ≤βΕ®Θ§«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ

(1) ≤ΜΡή”ΟΆ≠ΥΩΫΝΑηΑτ¥ζΧφΜΖ–Έ≤ΘΝßΫΝΑηΑτΒΡάμ”… «_________ΓΘ
(2)»γ”Ο0.50 molΓΛLΘ≠1―ΈΥα”κNaOHΙΧΧεΫχ–– Β―ιΘ§‘ρ Β―ι÷–≤βΒΟΒΡΓΑ÷–ΚΆ»»Γ± ΐ÷ΒΫΪ________(ΧνΓΑΤΪ¥σΓ±ΓΔΓΑΤΪ–ΓΓ±ΓΔΓΑ≤Μ±δΓ±)
(3) Β―ιΒΟΒΫ±μ÷–ΒΡ ΐΨίΘΚ
|
Β―ι¥Έ ΐ |
Τπ ΦΈ¬Ε»t1/Γφ |
÷’÷ΙΈ¬Ε»t2/Γφ |
|
|
―ΈΥα |
NaOH»ή“Κ |
||
|
1 |
20.2 |
20.3 |
23.7 |
|
2 |
20.3 |
20.5 |
23.8 |
|
3 |
21.5 |
21.6 |
24.9 |
Ψ≠ ΐΨί¥ΠάμΘ§t2Θ≠t1ΘΫ3.4ΓφΓΘ‘ρΗΟ Β―ι≤βΒΟΒΡ÷–ΚΆ»» ΠΛH ΘΫ________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com