
| A�����Ը��������Һ | B������������Һ | C�����Ȼ�̼ | D�����軯����Һ |
| | ѡ���Լ�(����) | ʵ������ |
| ��һ�ַ��� | | |
| �ڶ��ַ��� | | |
| �����ַ��� | | |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1:1 | B��1:2 | C��2:1 | D��2:3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
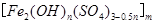 ���㷺������ˮ������ʵ�����������᳧��������Ҫ�ɷ�Ϊ���������P����FeS��SiO2�ȣ��Ʊ��������̷���FeSO4��7H2O ���������£�
���㷺������ˮ������ʵ�����������᳧��������Ҫ�ɷ�Ϊ���������P����FeS��SiO2�ȣ��Ʊ��������̷���FeSO4��7H2O ���������£�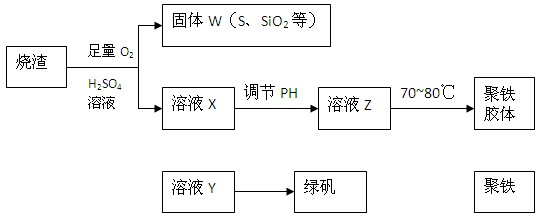
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ͭ�ڸ���Ŀ����к��ڳ�ʪ�Ŀ����д��ڵ���̬��ͬ |
| B����ͬ������ͭ�ֱ�������������������ȫ��Ӧ��ʧȥ�ĵ�������ͬ |
| C����ͬ���ʵ�����Ũ����ֱ���������ͭ������ͭ��Ӧ����������ͭ������ͬ |
| D����ͬ������ͭ�ֱ���������ϡ���ᡢŨ������ȫ��Ӧ��������������ʵ�����ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2��3��2 | B��3��3��2 | C��3��2��2 | D�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
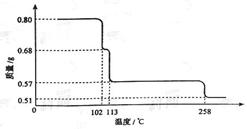
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A������ | B�������� | C�����Ժ������� | D����ԭ�� |
 ��mol��
��mol�� ol���ú�a��ʽ�ӱ�ʾ����
ol���ú�a��ʽ�ӱ�ʾ���� 2NaNO2��H2O
2NaNO2��H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
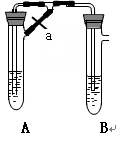
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com