

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ŌĖÓĆ»Æѧ·“Ó¦ŌĄķŃŠ¾æµŖ”¢ŃõµČµ„ÖŹ¼°Ęä»ÆŗĻĪļµÄ·“Ó¦ÓŠÖŲŅŖŅāŅ壮
ŌĖÓĆ»Æѧ·“Ó¦ŌĄķŃŠ¾æµŖ”¢ŃõµČµ„ÖŹ¼°Ęä»ÆŗĻĪļµÄ·“Ó¦ÓŠÖŲŅŖŅāŅ壮| 10-9 |
| a-0.01 |
| 10-9 |
| a-0.01 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
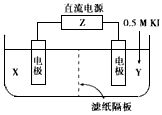
(11·Ö)µē½āŌĄķŌŚ»Æѧ¹¤ŅµÖŠÓŠ¹ć·ŗÓ¦ÓĆ”£ÓŅĶ¼±ķŹ¾Ņ»øöµē½ā³Ų£¬ĘäÖŠaĪŖµē½āÖŹČÜŅŗ£¬ X”¢YŹĒĮ½æéµē¼«°å£¬Ķعżµ¼ĻßÓėÖ±Į÷µēŌ“ĻąĮ¬”£Ēė»Ų“šŅŌĻĀĪŹĢā£ŗ
£Ø1£©XµÄµē¼«Ćū³ĘŹĒ ”£
£Ø2£©ČōX”¢Y¶¼ŹĒ¶čŠŌµē¼«£¬aŹĒĮņĖįÄĘČÜŅŗ£¬ŹµŃéæŖŹ¼Ź±£¬Ķ¬Ź±ŌŚĮ½±ßø÷µĪČė¼øµĪŹÆČļŹŌ¼Į£¬Ņ»¶ĪŹ±¼äŗó£¬ŌŚX¼«ø½½ü¹Ū²ģµ½µÄĻÖĻóŹĒ £¬Y¼«ÉĻµÄµē¼«·“Ó¦Ź½ĪŖ ”£
£Ø3£©ČōX”¢Y¶¼ŹĒ¶čŠŌµē¼«£¬aŹĒCuSO4ČÜŅŗ£¬µē½āŅ»¶ĪŹ±¼äŗó£¬Ńō¼«ÉĻ²śÉśĘųĢåµÄĢå»żĪŖ0.224L£Ø±ź×¼×“æöĻĀ£©£¬ŌņŅõ¼«ÉĻĪö³ö½šŹōµÄÖŹĮæĪŖ g”£
£Ø4£©ČōŅŖÓĆøĆ×°ÖƵē½ā¾«Į¶“ÖĶ£¬µē½āŅŗaŃ”ÓĆCuSO4ČÜŅŗ£¬ŌņXµē¼«µÄ²ÄĮĻŹĒ
£¬Yµē¼«µÄ²ÄĮĻŹĒ ”£
£Ø5£©ČōŅŖÓƵē¶Ę·½·ØŌŚĢś±ķĆę¶ĘŅ»²ć½šŹōŅų£¬Ó¦øĆŃ”ŌńµÄ·½°øŹĒ ”£
| ·½°ø | X | Y | aČÜŅŗ |
| A | Ņų | ŹÆÄ« | AgNO3 |
| B | Ņų | Ģś | AgNO3 |
| C | Ģś | Ņų | Fe(NO3)3 |
| D | Ģś | Ņų | AgNO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğø£½ØŹ”°²ĻŖ”¢»Ż°²”¢ŃųÕżÖŠŃ§ø߶žÉĻѧʌʌ֊ĮŖæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
(11·Ö)µē½āŌĄķŌŚ»Æѧ¹¤ŅµÖŠÓŠ¹ć·ŗÓ¦ÓĆ”£ÓŅĶ¼±ķŹ¾Ņ»øöµē½ā³Ų£¬ĘäÖŠaĪŖµē½āÖŹČÜŅŗ£¬ X”¢YŹĒĮ½æéµē¼«°å£¬Ķعżµ¼ĻßÓėÖ±Į÷µēŌ“ĻąĮ¬”£Ēė»Ų“šŅŌĻĀĪŹĢā£ŗ
£Ø1£©XµÄµē¼«Ćū³ĘŹĒ ”£
£Ø2£©ČōX”¢Y¶¼ŹĒ¶čŠŌµē¼«£¬aŹĒĮņĖįÄĘČÜŅŗ£¬ŹµŃéæŖŹ¼Ź±£¬Ķ¬Ź±ŌŚĮ½±ßø÷µĪČė¼øµĪŹÆČļŹŌ¼Į£¬Ņ»¶ĪŹ±¼äŗó£¬ŌŚX¼«ø½½ü¹Ū²ģµ½µÄĻÖĻóŹĒ £¬Y¼«ÉĻµÄµē¼«·“Ó¦Ź½ĪŖ ”£
£Ø3£©ČōX”¢Y¶¼ŹĒ¶čŠŌµē¼«£¬aŹĒCuSO4ČÜŅŗ£¬µē½āŅ»¶ĪŹ±¼äŗó£¬Ńō¼«ÉĻ²śÉśĘųĢåµÄĢå»żĪŖ0.224L£Ø±ź×¼×“æöĻĀ£©£¬ŌņŅõ¼«ÉĻĪö³ö½šŹōµÄÖŹĮæĪŖ g”£
£Ø4£©ČōŅŖÓĆøĆ×°ÖƵē½ā¾«Į¶“ÖĶ£¬µē½āŅŗaŃ”ÓĆCuSO4ČÜŅŗ£¬ŌņXµē¼«µÄ²ÄĮĻŹĒ
£¬Yµē¼«µÄ²ÄĮĻŹĒ ”£
£Ø5£©ČōŅŖÓƵē¶Ę·½·ØŌŚĢś±ķĆę¶ĘŅ»²ć½šŹōŅų£¬Ó¦øĆŃ”ŌńµÄ·½°øŹĒ ”£
| ·½°ø | X | Y | aČÜŅŗ |
| A | Ņų | ŹÆÄ« | AgNO3 |
| B | Ņų | Ģś | AgNO3 |
| C | Ģś | Ņų | Fe(NO3)3 |
| D | Ģś | Ņų | AgNO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011ÄźĘÕĶØøßµČѧŠ£ÕŠÉśČ«¹śĶ³Ņ»æ¼ŹŌ»Æѧ¾ķ£Ø½ĖÕ£© ĢāŠĶ£ŗĢīæÕĢā
ĒāĘųŹĒŅ»ÖÖĒå½ąÄÜŌ“£¬ĒāĘųµÄÖĘČ”Óė“¢“ęŹĒĒāÄÜŌ“ĄūÓĆĮģÓņµÄŃŠ¾æČČµć”£
ŅŃÖŖ£ŗ £Øg£©+
£Øg£©+  £Øg£©====
£Øg£©==== £Øg£©+
£Øg£©+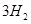 £Øg£©
£Øg£©  =
=


 £Øg£©+
£Øg£©+ 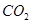 £Øg£©====
£Øg£©==== £Øg£©+
£Øg£©+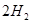 £Øg£©
£Øg£© =
= 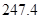

 £Øg£©====
£Øg£©====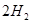 £Øg£©+
£Øg£©+ £Øg£©
£Øg£©  =
=


£Ø1£©ŅŌ¼×ĶéĪŖŌĮĻÖĘČ”ĒāĘųŹĒ¹¤ŅµÉĻ³£ÓƵÄÖĘĒā·½·Ø”£ £Øg£©Óė
£Øg£©Óė £Øg£©·“Ӧɜ³É
£Øg£©·“Ӧɜ³É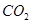 £Øg£©ŗĶ
£Øg£©ŗĶ £Øg£©µÄČČ»Æѧ·½³ĢŹ½ĪŖ______”£
£Øg£©µÄČČ»Æѧ·½³ĢŹ½ĪŖ______”£
£Ø2£© ČČ·Ö½āÖĘĒāŹ±£¬³£Ļņ·“Ó¦Ę÷ÖŠĶØČėŅ»¶Ø±ČĄżæÕĘų£¬Ź¹²æ·Ö
ČČ·Ö½āÖĘĒāŹ±£¬³£Ļņ·“Ó¦Ę÷ÖŠĶØČėŅ»¶Ø±ČĄżæÕĘų£¬Ź¹²æ·Ö Č¼ÉÕ£¬ĘäÄæµÄŹĒ_____;Č¼ÉÕÉś³ÉµÄ
Č¼ÉÕ£¬ĘäÄæµÄŹĒ_____;Č¼ÉÕÉś³ÉµÄ Óė
Óė ½ųŅ»²½·“Ó¦£¬Éś³ÉĪļŌŚ³£ĪĀĻĀ¾ł·ĒĘųĢ壬Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ_______”£
½ųŅ»²½·“Ó¦£¬Éś³ÉĪļŌŚ³£ĪĀĻĀ¾ł·ĒĘųĢ壬Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ_______”£
£Ø3£©H OµÄČČ·Ö½āŅ²æɵƵ½H
OµÄČČ·Ö½āŅ²æɵƵ½H £¬øßĪĀĻĀĖ®·Ö½āĢåĻµÖŠÖ÷ŅŖĘųĢåµÄĢå»ż·ÖŹżÓėĪĀ¶ČµÄ¹ŲĻµČēĶ¼11ĖłŹ¾”£Ķ¼ÖŠA”¢B±ķŹ¾µÄĪļÖŹŅĄ“ĪŹĒ_______”£
£¬øßĪĀĻĀĖ®·Ö½āĢåĻµÖŠÖ÷ŅŖĘųĢåµÄĢå»ż·ÖŹżÓėĪĀ¶ČµÄ¹ŲĻµČēĶ¼11ĖłŹ¾”£Ķ¼ÖŠA”¢B±ķŹ¾µÄĪļÖŹŅĄ“ĪŹĒ_______”£

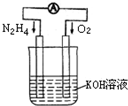
£Ø4£©µē½āÄņĖŲ[CO(NH )
) ]µÄ¼īŠŌČÜŅŗÖĘĒāµÄ×°ÖĆŹ¾ŅāĶ¼¼ūĶ¼12£Øµē½ā³ŲÖŠøōĤ½ö×čÖ¹ĘųĢåĶعż£¬Ņõ”¢Ńō¼«¾łĪŖ¶čŠŌµē¼«£©”£µē½āŹ±£¬Ńō¼«µÄµē¼«·“Ó¦Ź½ĪŖ_______”£
]µÄ¼īŠŌČÜŅŗÖĘĒāµÄ×°ÖĆŹ¾ŅāĶ¼¼ūĶ¼12£Øµē½ā³ŲÖŠøōĤ½ö×čÖ¹ĘųĢåĶعż£¬Ņõ”¢Ńō¼«¾łĪŖ¶čŠŌµē¼«£©”£µē½āŹ±£¬Ńō¼«µÄµē¼«·“Ó¦Ź½ĪŖ_______”£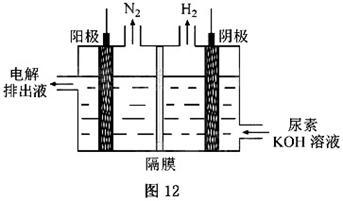
£Ø5£©Mg CuŹĒŅ»ÖÖ“¢ĒāŗĻ½š”£350”ꏱ£¬Mg
CuŹĒŅ»ÖÖ“¢ĒāŗĻ½š”£350”ꏱ£¬Mg CuÓėH
CuÓėH ·“Ó¦£¬Éś³ÉMgCu
·“Ó¦£¬Éś³ÉMgCu ŗĶ½öŗ¬Ņ»ÖÖ½šŹōŌŖĖŲµÄĒā»ÆĪļ£ØĘäÖŠĒāµÄÖŹĮæ·ÖŹżĪŖ0.077£©”£Mg
ŗĶ½öŗ¬Ņ»ÖÖ½šŹōŌŖĖŲµÄĒā»ÆĪļ£ØĘäÖŠĒāµÄÖŹĮæ·ÖŹżĪŖ0.077£©”£Mg CuÓėH
CuÓėH ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_______”£
·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_______”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com