
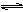 CH3CH2OH(g)+3H2O(g) ”÷H£½a kJ”¤mol£1
CH3CH2OH(g)+3H2O(g) ”÷H£½a kJ”¤mol£1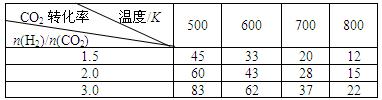
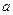 0(Ģī”°“óÓŚ”±»ņ”°Š”ÓŚ”±)”£
0(Ģī”°“óÓŚ”±»ņ”°Š”ÓŚ”±)”£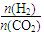 )±Č£¬Ę½ŗā³£ŹżKÖµ £ØĢī”°Ōö“ó”±”¢”°¼õŠ””±”¢»ņ”°²»±ä”±£©£¬¶ŌÉś³ÉŅŅ“¼ £ØĢī”°ÓŠĄū”±»ņ”°²»Ąū”±£©”£
)±Č£¬Ę½ŗā³£ŹżKÖµ £ØĢī”°Ōö“ó”±”¢”°¼õŠ””±”¢»ņ”°²»±ä”±£©£¬¶ŌÉś³ÉŅŅ“¼ £ØĢī”°ÓŠĄū”±»ņ”°²»Ąū”±£©”£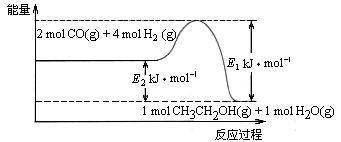
 ¾ŁŅ»·“Čżµ„ŌŖĶ¬²½¹ż¹Ų¾ķĻµĮŠ“š°ø
¾ŁŅ»·“Čżµ„ŌŖĶ¬²½¹ż¹Ų¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
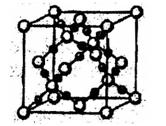
| A£®ŌŚSiO2¾§ĢåÖŠ£¬ĆæøöSiŌ×ÓÓė4øöOŌ×ÓŠĪ³É¹²¼Ū¼ü |
| B£®ŌŚĆęŠÄĮ¢·½ĆܶѻżµÄ½šŹō¾§ĢåÖŠ£¬Ćæøö½šŹōŌ×ÓÖÜĪ§½ōĮŚµÄÓŠ4øö½šŹōŌ×Ó |
| C£®NaCl¾§ĢåÖŠÓėĆæøöNa+¾ąĄėĻąµČĒŅ×ī½üµÄCl”ŖÓŠ6øö£» |
| D£®CsCl¾§ĢåÖŠÓėĆæøöCs+¾ąĄėĻąµČĒŅ×ī½üµÄCl”ŖÓŠ8øö |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŌŖĖŲµÄ·Ē½šŹōŠŌŌ½Ē棬Ę䵄֏µÄ»īĘĆŠŌŅ»¶ØŌ½Ēæ |
| B£®Ō×Ó¾§ĢåÖŠŌ×ÓŅŌ¹²¼Ū¼ü½įŗĻ£¬¾ßÓŠ¼üÄÜ“ó”¢ČŪµćøß”¢Ó²¶Č“óµÄĢŲŠŌ |
| C£®·Ö×Ó¾§ĢåµÄČŪ·ŠµćµĶ£¬³£ĪĀĻĀ¾ł³ŹŅŗĢ¬»ņĘųĢ¬ |
| D£®ŗ¬ÓŠ½šŹōŃōĄė×ӵľ§ĢåŅ»¶ØŹĒĄė×Ó¾§Ģå |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| Ō×ÓŠņŹż | Ō×Ó¼Ū²ćµē×ÓÅŲ¼ | ÖÜĘŚ | ×å |
| 17 | ¢Ł | µŚČż | ¢Ś |
| ¢Ū | 3d54s1 | ¢Ü | ¢öB |
 2s
2s 2p
2p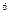 3s
3s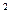 3p
3p 4s
4s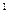 £¬ŌņøĆŌŖĖŲ»łĢ¬Ō×ӵĵē×ÓÅŲ¼Ź½ĪŖ £»Ęä×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļµÄ»ÆѧŹ½ŹĒ ”£
£¬ŌņøĆŌŖĖŲ»łĢ¬Ō×ӵĵē×ÓÅŲ¼Ź½ĪŖ £»Ęä×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļµÄ»ÆѧŹ½ŹĒ ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®KCl”¢ H2SO4”¢S | B£®½šøÕŹÆ”¢NH4Cl”¢CH4 |
| C£®HF”¢ SiO2”¢ Al | D£®½šøÕŹÆ”¢SiO2”¢Na2CO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā

| A£®¾§ĢåSi < ½šøÕŹÆ | B£®CO2 < SiO2 | C£® NaCl < NaBr | D£® PH3 < NH3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
|

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 2øöO2-Ļą½ōĮŚ
2øöO2-Ļą½ōĮŚ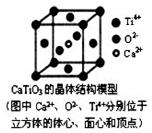
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
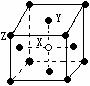
 s2np2£¬ĒŅXŌ×Ó°ė¾¶Š”ÓŚQ£»¢ŚYŌŖĖŲŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄŌŖĖŲ£»WŌŖĖŲµÄµēøŗŠŌĀ·Š”ÓŚYŌŖĖŲ£¬ŌŚWŌ×ӵļŪµē×ÓÅŲ¼Ź½ÖŠ£¬p¹ģµĄÉĻÖ»ÓŠ1øöĪ“³É¶Ōµē×Ó£»¢ŪZŌŖĖŲµÄµēĄėÄÜŹż¾Ż¼ūĻĀ±ķ£ØkJ”¤mol-1£©
s2np2£¬ĒŅXŌ×Ó°ė¾¶Š”ÓŚQ£»¢ŚYŌŖĖŲŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄŌŖĖŲ£»WŌŖĖŲµÄµēøŗŠŌĀ·Š”ÓŚYŌŖĖŲ£¬ŌŚWŌ×ӵļŪµē×ÓÅŲ¼Ź½ÖŠ£¬p¹ģµĄÉĻÖ»ÓŠ1øöĪ“³É¶Ōµē×Ó£»¢ŪZŌŖĖŲµÄµēĄėÄÜŹż¾Ż¼ūĻĀ±ķ£ØkJ”¤mol-1£©| I1 | I2 | I3 | I4 | ” |
| 496 | 4562 | 6912 | 9540 | ” |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com