

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
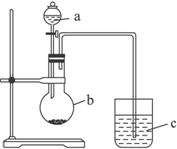
(1)����a��������__________��Ӧʢ������ҩƷ�е�__________��
A.ϡ���� B.������ C.������ D.����
(2)����b��������__________��Ӧʢ������ҩƷ�е�__________��
A.̼��� B.������ C.�Ȼ��� D.̼����
(3)����c��Ӧʢ�ŵ�ҩƷ��_________�����������������_________��֤��b�з�Ӧ�����ˣ�����˵��__________��__________����ǿ���ǽ�����__________��__________ǿ��b�з�����Ӧ�����ӷ���ʽΪ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ��ͨ���Ƚ�������������������ǿ������֤����̼�ķǽ����Ե�ǿ����������������ͼ��װ�á���ش�

(1)����a��������__________��Ӧʢ������ҩƷ�е�__________��
A.ϡ���� B.������ C.������ D.����
(2)����b��������__________��Ӧʢ������ҩƷ�е�__________��
A.̼��� B.������ C.�Ȼ��� D.̼����
(3)����c��Ӧʢ�ŵ�ҩƷ��_________�����������������_________��֤��b�з�Ӧ�����ˣ�����˵��__________��__________����ǿ���ǽ�����__________��__________ǿ��b�з�����Ӧ�����ӷ���ʽΪ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ��ͨ���Ƚ�������������������ǿ������֤����̼�ķǽ����Ե�ǿ����������������ͼ��װ�á���ش�

(1)����a��������__________��Ӧʢ������ҩƷ�е�__________��
A.ϡ���� B.������ C.������ D.����
(2)����b��������__________��Ӧʢ������ҩƷ�е�__________��
A.̼��� B.������ C.�Ȼ��� D.̼����
(3)����c��Ӧʢ�ŵ�ҩƷ��_________�����������������_________��֤��b�з�Ӧ�����ˣ�����˵��__________��__________����ǿ���ǽ�����__________��__________ǿ��b�з�����Ӧ�����ӷ���ʽΪ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�Ƹ���ѧ����11���¿���ѧ���Ծ� ���ͣ�ʵ����
��5�֣�ijͬѧ��ͨ���Ƚ���������������Ӧˮ���������ǿ������֤����̼�ķǽ����Ե�ǿ��������������ͼ��ʾ��װ�á���ش�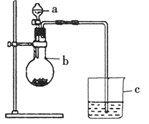
��1������a�������� ��Ӧʢ������ҩƷ�е� ��
A��ϡ���� B��������
C�������� D������
��2������b�������� ��Ӧʢ������ҩƷ�е� ��
A��̼��� B��������
C���Ȼ��� D��̼����
��3������۲쵽C�е�����Ϊ ������֤������̼�ķǽ����Ե�ǿ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com