
| A£®µČĢå»żµČĪļÖŹµÄĮæÅØ¶ČµÄNaClO(aq)ÓėNaCl(aq)ÖŠĄė×Ó×ÜŹż“󊔣ŗNĒ°£¾Nŗó |
B£®0.1 mol”¤L£1Na2SČÜŅŗÖŠ£ŗ2c(Na£«) c(S2£)£«c(HS£)£«c(H2S) c(S2£)£«c(HS£)£«c(H2S) |
| C£®³£ĪĀĻĀ½«“×ĖįÄĘ”¢ŃĪĖįĮ½ČÜŅŗ»ģŗĻŗó£¬ČÜŅŗ³ŹÖŠŠŌ£¬Ōņ»ģŗĻŗóČÜŅŗÖŠ£ŗ c(Na£«)£¾c(Cl”Ŗ)£¾c(CH3COOH) |
| D£®0.1 mol”¤L£1Na2CO3ČÜŅŗÓė0.1 mol”¤L£1NaHCO3ČÜŅŗµČĢå»ż»ģŗĻ |
 c(HCO3£ )£«3c(H2CO3)£«2c(H£«)
c(HCO3£ )£«3c(H2CO3)£«2c(H£«) c(ClO£)£«c(OH£)”¢c(Na£«)£«c(H£«)
c(ClO£)£«c(OH£)”¢c(Na£«)£«c(H£«) c(Cl£)£«c(OH£)£¬ÓÉÓŚNaClOĖ®½āĻŌ¼īŠŌ£¬Ņņ“ĖÓŠc(H£«)NaClO£¼c(H£«)NaCl£¬ĖłŅŌNNaClO£¼NNaCl£¬A“ķ”£Ń”ĻīBÖŠµÄĪļĮĻŹŲŗćŹ½Ó¦ĪŖ
c(Cl£)£«c(OH£)£¬ÓÉÓŚNaClOĖ®½āĻŌ¼īŠŌ£¬Ņņ“ĖÓŠc(H£«)NaClO£¼c(H£«)NaCl£¬ĖłŅŌNNaClO£¼NNaCl£¬A“ķ”£Ń”ĻīBÖŠµÄĪļĮĻŹŲŗćŹ½Ó¦ĪŖ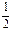 c(Na£«)
c(Na£«) c(S2£)£«c(HS£)£«c(H2S)”£Ń”ĻīCæÉŅŌÕāŃł·ÖĪö£ŗĻČŠ“³ö»ģŗĻČÜŅŗµÄµēŗÉŹŲŗćŹ½£ŗc(H£«)£«c(Na£«)
c(S2£)£«c(HS£)£«c(H2S)”£Ń”ĻīCæÉŅŌÕāŃł·ÖĪö£ŗĻČŠ“³ö»ģŗĻČÜŅŗµÄµēŗÉŹŲŗćŹ½£ŗc(H£«)£«c(Na£«) c(OH£)£« c(Cl”Ŗ)£« c(CH3COO£)£¬ÓÖÓÉÓŚČÜŅŗ³ŹÖŠŠŌ¼“c(H£«)£½c(OH£)£¬Ņņ“Ė»ģŗĻČÜŅŗÖŠÓ¦ÓŠc(Na£«)
c(OH£)£« c(Cl”Ŗ)£« c(CH3COO£)£¬ÓÖÓÉÓŚČÜŅŗ³ŹÖŠŠŌ¼“c(H£«)£½c(OH£)£¬Ņņ“Ė»ģŗĻČÜŅŗÖŠÓ¦ÓŠc(Na£«) c(Cl”Ŗ)£«c(CH3COO£)£¬øł¾ŻĪļĮĻŹŲŗćæɵĆc(Na£«)
c(Cl”Ŗ)£«c(CH3COO£)£¬øł¾ŻĪļĮĻŹŲŗćæɵĆc(Na£«) c(CH3COOH)£«c(CH3COO£)£¬ĖłŅŌÓŠc(Na£«)£¾c(Cl”Ŗ)
c(CH3COOH)£«c(CH3COO£)£¬ĖłŅŌÓŠc(Na£«)£¾c(Cl”Ŗ) c(CH3COOH)£¬C“ķ”£
c(CH3COOH)£¬C“ķ”£

 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®CuOÓėĻ”H2SO4·“Ó¦ CuO+2H+=Cu2++H2O |
| B£®½šŹōÄĘĶ¶Čėµ½Ė®ÖŠ Na+2H2O=Na++2OH-+H2”ü |
| C£®CuSO4ČÜŅŗÓėBa(OH)2ČÜŅŗ·“Ó¦ SO42-+Ba2+=BaSO4”ż |
| D£®×ćĮæNaOHČÜŅŗÓėÉŁĮæCO2·“Ó¦2OH-+CO2=CO32-+H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| ŃōĄė×Ó | K+ Ba2+ Ag+ Mg2+ NH4+ Na+ |
| ŅõĄė×Ó | SO42£ SO32£ CO32£ AlO2£ |
| ÅųżµÄĄė×Ó | ÅųżµÄŅĄ¾Ż |
| | |
| æĻ¶Ø“ęŌŚµÄĄė×Ó | ÅŠ¶ĻŅĄ¾Ż |
| | |
| ÅųżµÄĄė×Ó | ÅųżµÄŅĄ¾Ż |
| | |
| ÉŠ“ż¼ģŃéµÄĄė×Ó | ¼ģŃé·½·Ø |
| | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®pH=3µÄHNO3ÓėpH=11µÄKOHČÜŅŗ |
| B£®pH=3µÄHNO3ÓėpH=11µÄ°±Ė® |
| C£®pH=3µÄH2SO4ÓėpH=11µÄNaOHČÜŅŗ |
| D£®pH=3µÄCH3COOHÓėpH=11µÄBa(OH)2ČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®c£ØCH3COO££©£ŗ¢Ū£¾¢Ł |
| B£®Ė®µēĄė³öµÄc£ØOH££©£ŗ¢Ś£¾¢Ł |
C£®¢ŁŗĶ¢ŚµČĢå»ż»ģŗĻŗóµÄČÜŅŗ£ŗ c£ØOH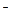 £©£½c£ØH+£©+ c£ØCH3COOH£© £©£½c£ØH+£©+ c£ØCH3COOH£© |
| D£®¢ŁŗĶ¢ŪµČĢå»ż»ģŗĻŗóµÄČÜŅŗ£ŗ c£ØNa+£©£¾c£ØCH3COO-£©£¾c£ØH+£©£¾c£ØOH-£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 ӢCl-ӢNH
”¢Cl-”¢NH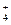 B£®Ba2£«”¢Mg2£«”¢NO
B£®Ba2£«”¢Mg2£«”¢NO ”¢Cl-
”¢Cl-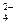 ”¢Cl-”¢K£«”¢NH
”¢Cl-”¢K£«”¢NH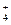 D£®SO
D£®SO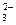 ”¢K£«”¢AlO
”¢K£«”¢AlO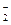 ”¢Na£«
”¢Na£«²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 ø÷×éĄė×ÓÄÜ“óĮæ¹²“ęµÄŹĒ
ø÷×éĄė×ÓÄÜ“óĮæ¹²“ęµÄŹĒA£®pH£½14µÄČÜŅŗ£ŗNa£«”¢Ba2£«”¢AlO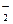 ”¢ClO£ ”¢ClO£ |
B£®¼ÓČėAl·Å³öH2µÄČÜŅŗ£ŗCu2£«”¢NH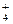 ”¢Cl£”¢NO ”¢Cl£”¢NO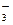 |
C£®Ź¹×ĻÉ«ŹÆČļŹŌŅŗ±äĄ¶µÄČÜŅŗ£ŗK£«”¢Fe3£«”¢Cl£”¢NO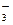 |
D£®Ź¹¼×»ł³ČŹŌŅŗ±äŗģµÄČÜŅŗ£ŗFe2£«”¢Al3£«”¢Cl£”¢NO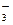 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com