
�����£�Ksp(AgCl)=1.8��10-10, Ksp(AgI)=1.0��10-14,���������AgCl��AgI�ı�����Һ�����ϣ��������м���һ������AgNO3���壬����˵����ȷ����
A. ��AgI��Һ�м���AgNO3��c(Ag+)����Ksp(AgI)Ҳ����
B. ����Һ��ϣ�AgCl��AgI������
C. ��ȡ0.1435gAgCl�������100mLˮ����������仯����c(Cl-)Ϊ0.01mol/L
D. ��AgNO3������AgCl��AgI���ɳ���������AgClΪ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ������ѧ��������2����ѧ�Ծ��������棩 ���ͣ�ѡ����
��������X����ͨ��Y��Һ�У�ʵ������Ԥ�������һ�µ������
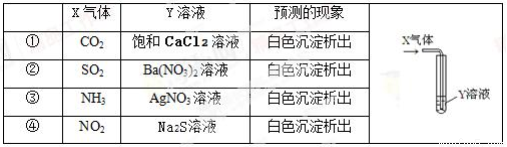
A. ֻ�Т٢ڢ� B. ֻ�Т٢� C. ֻ�Т� D. �ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и����ڶ��δ��������ۻ�ѧ�Ծ��������棩 ���ͣ������
�軯�أ�KCN����һ���о綾�����ʣ������ʹ��ʱ����ע�ⰲȫ����֪��KCN+H2O2=KOCN+H2O���ش��������⣺
��1��OCN-����������Ԫ�صĵ�һ�����ܴӴ�С��˳��Ϊ_________����Ԫ�ط��ű�ʾ����ͬ�����縺�ԴӴ�С��˳��Ϊ________����̬��ԭ����Χ�����Ų�ʽΪ__________��
��2��H2O2�еĹ��ۼ�����Ϊ_______����Ҽ����м���) ��������ԭ�ӵ��ӻ��������Ϊ_________��������4��ԭ��______����ڡ����ڡ���ͬһ��ֱ���ϣ�H2O2������ˮ�����Ƕ��Ǽ��Է����⣬����Ϊ____________________��
��3����OCN-���Ϸ�ʽ��ͬ�һ�Ϊ�ȵ�����ķ���Ϊ________���ξ�һ����������OCN-��Ϊ�ȵ���������У���һ��Ԫ����ɵ���������____________��
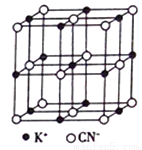
��4��KCN�ľ����ṹ��ͼ��ʾ��������K������λ��Ϊ_______�����侧������a=0.648nm����KCN ������ܶ�Ϊ_______g/cm3���г�����ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�켪��ʡ�����и�����ѧ�ڵڶ�������������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
ʹ�����͵缫���ϣ���N2��H2Ϊ�缫��Ӧ���HC1-NH4C1Ϊ�������Һ���������һ�ּ����ṩ���ܣ�����ʵ�ֵ��̶�������ȼ�ϵ�أ�ԭ������ͼ��ʾ�������йط�����ȷ���ǣ� ��
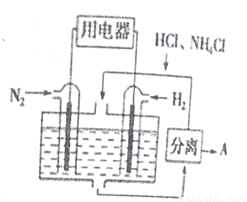
A. ͨ��H2����Ϊ���� B. �����������XΪHC1
C. ��ع���һ��ʱ�����ҺpH��С D. ͨ��N2һ���ĵ缫��ӦʽΪ��N2+6e-+8H+=2NH4+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ���������е�ʮ��У�ص���ѧ������һ��������ѧ�Ծ��������棩 ���ͣ�ʵ����
������(AlN)��һ���������ǽ������ϡ�Ϊ�˷���ijAlN��Ʒ����Ʒ�е����ʲ���NaOH��Һ��Ӧ���� AlN�ĺ�����ijʵ��С���������������ʵ�鷽������֪��AlN+NaOH+H2O��NaAlO2+NH3��
������1��ȡһ��������Ʒ��������װ�òⶨ��Ʒ��AlN�Ĵ���(�г�װ������ȥ)��
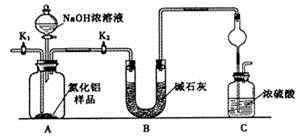
��1����ͼװ���У�U�ι�B����װ����Ϊ________��C�����θ���ܵ�������_______________________��
��2���ر�K1��K2���ٴ�Һ©������������NaOHŨ��Һ�������ٲ������塣��K1��ͨ�뵪��һ��ʱ�䣬�ⶨCװ�÷�Ӧǰ��������仯��ͨ�뵪����Ŀ����_______________________________________��
��3����������װ�û�����____________ȱ�ݣ����²ⶨ���ƫ�ߡ�
������2�������²���ⶨ��Ʒ��AlN�Ĵ��ȣ�
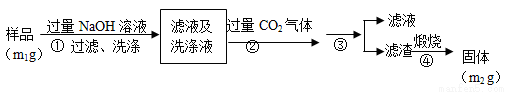
��4����������ɳ��������ӷ���ʽΪ___________________��
��5������۵IJ������õ�����Ҫ����������_________��AlN�Ĵ�����__________����m1��m2��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ���������е�ʮ��У�ص���ѧ������һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪�л���A(C10H20O2)�������������ܹ�ˮ���B��C, B��һ�������¿��������õ�C��������������л���A��
A. 4�� B. 8�� C. 16�� D. 32��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ���������е�ʮ��У�ص���ѧ������һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
��ͼ��ʾ����������ʵ�����Ʊ�������ˮFeCl3����������˳����ȷ����
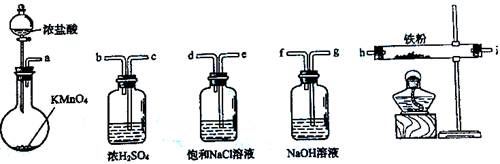
A. a-b-c-d-e-e-f-g-h B. a-e-d-c-b-h-i-g
C. a-d-e-c-b-h-i-g D. a-c-b-d-e-h-i-f
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�߶�3��ѧҵˮƽ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
һ���¶��£����ݻ�������ܱ������з������·�Ӧ��I2(g) + H2(g) 2HI(g)���÷�Ӧ�ﵽƽ��״̬�ı�־�ǣ� ��
2HI(g)���÷�Ӧ�ﵽƽ��״̬�ı�־�ǣ� ��
A. �������������ɫ���ٸı�
B. I2��H2��HI�ķ�����֮��Ϊ1��1��2
C. I2(g) ��H2(g)��ȫת��Ϊ HI(g)
D. ��λʱ��������n mol I2��ͬʱ����n mol H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017���Ĵ�ʡ������ѧ�ڵ��Ĵ��¿������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
���ܵĴ洢������Ӧ�õ���Ҫƿ������λ�⻯��������廯������Ŀǰ�����õ���Ҫ������ϡ�
��1��Ti��BH4��2��һ�ֹ���Ԫ�����⻯�ﴢ����ϡ��ڻ�̬Ti�У��۵����Ų�ʽΪ ____���۵����Ų�ͼΪ____
��2��Һ���Ǹ������ʣ������ܵ��������壬����N2+3H2 2NH3,ʵ�ִ�������⡣����˵����ȷ����_____ ��
2NH3,ʵ�ִ�������⡣����˵����ȷ����_____ ��
a��NH3�����е�ԭ�ӵĹ���ӻ���ʽΪsp2�ӻ�
b��NH+4��PH+4��CH4��BH-4��ClO��4��Ϊ�ȵ�����
c����ͬѹǿʱ��NH3�ķе��PH3�ķе��
d��[Cu��NH3��4]2+�����У�Nԭ������λԭ��
��3���ü۲���ӶԻ��������ƶ�SnBr2�����У�Snԭ�ӵĹ���ӻ���ʽΪ__________��
SnBr2������ Sn-Br�ļ���______120��(�������������=��)��
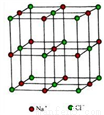
(4) NiO �ľ���ṹ���Ȼ�����ͬ�� �ھ����������ӵ���λ����_______��
��֪�����ı߳�Ϊ a nm�� NiO ��Ħ������Ϊ b g��mol-1�� NAΪ�����ӵ�������ֵ�� ��NiO ������ܶ�Ϊ_________g��cm-3��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com