
Ba(NO3)2æÉÓĆÓŚÉś²śĀĢÉ«ŃĢ»Ø”¢ĀĢÉ«ŠÅŗŵƔ¢ÕØŅ©”¢ĢÕ“ÉÓŌŅ©µČ”£±µŃĪÉś²śÖŠÅųö“óĮæµÄ±µÄą[Ö÷ŅŖŗ¬ÓŠBaCO3”¢BaSO3”¢Ba (FeO2)2µČ]£¬Ä³Ö÷ŅŖÉś²śBaCO3”¢BaSO4µÄ»Æ¹¤³§ĄūÓƱµÄąÖĘČ”Ba(NO3)2¾§Ģå£Ø²»ŗ¬½į¾§Ė®£©£¬Ęä²æ·Ö¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
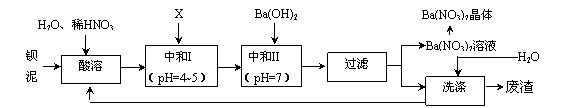
ŅŃÖŖ£ŗ¢ŁFe3+ŗĶFe2+ŅŌĒāŃõ»ÆĪļŠĪŹ½³ĮµķĶźČ«Ź±£¬ČÜŅŗµÄpH·Ö±šĪŖ3.2ŗĶ9.7£»
¢ŚBa(NO3)2¾§ĢåµÄ·Ö½āĪĀ¶Č£ŗ592”ę£»
¢ŪkSP(BaSO4)=l.l”Įl 0-10£¬kSP (BaCO3)=5 .l”Įl0-9”£
£Ø1£©øĆ³§Éś²śµÄBaCO3Ņņŗ¬ÓŠÉŁĮæBaSO4¶ų²»“棬Ģį“æµÄ·½·ØŹĒ£ŗ½«²śĘ·¼ÓČė×ćĮæµÄ±„ŗĶNa2CO3ČÜŅŗÖŠ£¬³ä·Ö½Į°č£¬¹żĀĖ£¬Ļ“µÓ”£ŹŌÓĆĄė×Ó·½³ĢŹ½ĖµĆ÷Ģį“æŌĄķ_____________________________________________________________”£
£Ø2£©ÉĻŹöĮ÷³ĢÖŠĖįČÜŹ±£¬Ba(FeO2)2ÓėHNO3·“Ӧɜ³ÉĮ½ÖÖĻõĖįŃĪ£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_______________________________________________”£
£Ø3£©øĆ³§½įŗĻŹµ¼Ź£¬Ķ¼ÖŠXӦєÓĆ_____________£ØĢī×ÖÄø£©”£
A£®BaCl2 B£®BaCO3 C£®Ba(NO3) 2 D£®Ba(OH) 2
£Ø4£©ÖŠŗĶIŹ¹ČÜŅŗµÄpHĪŖ4”«5µÄÄæµÄŹĒ________________________________£»½įŗĻĄė×Ó·½³ĢŹ½¼ņŹöĘäŌĄķ£ŗ________________________________”£
£Ø5£©“ÓBa(NO3)2ČÜŅŗÖŠ»ńµĆĘ侧ĢåµÄ²Ł×÷·½·ØŹĒ_____________________”£
£Ø6£©²ā¶ØĖłµĆBa(NO3)2¾§ĢåµÄ“æ¶Č£ŗ×¼Č·³ĘČ”wg¾§ĢåČÜÓŚÕōĮóĖ®£¬¼ÓČė×ćĮæµÄĮņĖį£¬³ä·Ö·“Ó¦ŗ󣬹żĀĖ”¢Ļ“µÓ”¢øÉŌļ£¬³ĘĮæĘäÖŹĮæĪŖm g£¬ŌņøĆ¾§ĢåµÄ“æ¶ČĪŖ_________________”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ļņ50 mL Na2SO4ŗĶNa2CO3µÄ»ģŗĻČÜŅŗÖŠ¼ÓČė¹żĮæµÄBaCl2ČÜŅŗ£¬µĆµ½14.51 g°×É«³Įµķ£¬Ļņ°×É«³ĮµķÖŠ¼ÓČė¹żĮæµÄĻ”HNO3£¬³ä·Ö·“Ó¦ŗ󣬳Įµķ¼õÉŁµ½4.66 g£¬²¢ÓŠĘųĢå²śÉś”£
£Ø1£©Ō»ģŗĻČÜŅŗÖŠNa2SO4ŗĶNa2CO3µÄĪļÖŹµÄĮæÅضČø÷ŹĒ¶ąÉŁ£æ
£Ø2£©²śÉśµÄĘųĢåµÄĪļÖŹµÄĮæĪŖ¶ąÉŁ£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½¹ŃĒĮņĖįÄĘ£ØNa2S2O5£©³£ÓĆ×÷Ź³Ę·ĘÆ°×¼Į”£ĘäÖʱø¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
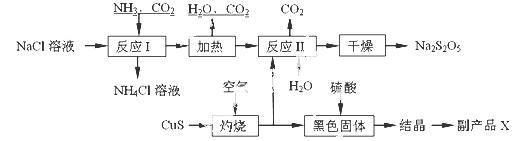
ŅŃÖŖ£ŗ·“Ó¦¢ņ°üŗ¬2NaHSO3 Na2S2O5£«H2OµČ¶ą²½·“Ó¦”£
Na2S2O5£«H2OµČ¶ą²½·“Ó¦”£
£Ø1£©ŹµŃéŹŅÖĘČ”°±ĘųµÄ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø2£©”°×ĘÉÕ”±Ź±·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø3£©ŅŃÖŖNa2S2O5ÓėĻ”ĮņĖį·“Ó¦·Å³öSO2£¬ĘäĄė×Ó·½³ĢŹ½ĪŖ£ŗ ”£
£Ø4£©ø±²śĘ·XµÄ»ÆѧŹ½ŹĒ£ŗ £»æÉŃ»·ĄūÓƵÄĪļÖŹŹĒ£ŗ__________________”£
£Ø5£©ĪŖĮĖ¼õÉŁ²śĘ·Na2S2O5ÖŠŌÓÖŹŗ¬Į棬ŠčæŲÖĘ·“Ó¦¢ņÖŠĘųĢåÓė¹ĢĢåµÄĪļÖŹµÄĮæÖ®±ČŌ¼
ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖÓɶĢÖÜĘŚŌŖĖŲ×é³ÉµÄĪļÖŹ A ”¢ B ”¢C ”¢D £¬¾ßÓŠČēĻĀ·“Ó¦¹ŲĻµ£ŗ
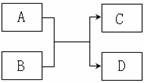 |
(1) Ķس£ČōAĪŖ»ĘĀĢÉ«µ„ÖŹ£¬BĪŖĪŽÉ«ŅŗĢ¬»ÆŗĻĪļ£¬ĒŅ0.1mol/LµÄCČÜŅŗpH=1£¬ŌņDĪļÖŹµÄ½į¹¹Ź½ĪŖ_____________
(2) ČōBĪŖ»ÆŗĻĪļ£¬ĒŅA”¢CĮ½ÖÖĪŽÉ«ĘųĢåĻąÓöŗó±äĪŖŗģ×ŲÉ«£¬ŌņBĪļÖŹČÜÓŚĖ®ŗóĻŌ¼īŠŌµÄŌŅņŹĒ__________________________________________________(ÓĆ»ÆѧÓĆÓļĖµĆ÷)
(3)ČōAĪŖµ„ÖŹ£¬CŗĶDĪŖ³£¼ūµÄŅ×Č¼ĘųĢ壬ŌņøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ____________________________________________________
(4) ČōAĪŖµ„ÖŹ£¬BĶس£ŹĒĪŽÉ«ŅŗĢ¬»ÆŗĻĪļ£¬×ĘÉÕD²śÉś»ĘÉ«»šŃę£¬Ōņ£ŗ
¢ŁČōB·Ö×Óŗ¬ÓŠ10øöµē×Ó£¬AÓėB·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ______________________________
¢ŚČōB·Ö×Óŗ¬ÓŠ18øöµē×Ó£¬ŌņA+B”śC+DµÄ»Æѧ·½³ĢŹ½ĪŖ_____________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŠšŹöÕżČ·µÄŹĒ
A£®ŠĀÖĘĀČĖ®ĻŌĖįŠŌ£¬ĻņĘäÖŠµĪ¼ÓÉŁĮæ×ĻÉ«ŹÆČļŹŌŅŗ£¬³ä·ÖÕńµ“ŗóČÜŅŗ³ŹŗģÉ«
B£®¼ÓĖ®Ļ”ŹĶČÜŅŗ£¬ČÜŅŗÖŠµÄĖłÓŠĄė×ÓÅØ¶Č¶¼¼õŠ”
C£®½šŹōĀĮµÄÉś²śŹĒŅŌAl2O3ĪŖŌĮĻ£¬ŌŚČŪȌדĢ¬ĻĀ½ųŠŠµē½ā
D£®NaHCO3ČÜŅŗÖŠŗ¬ÓŠÉŁĮæNa2CO3æÉŅŌÓĆ³ĪĒåŹÆ»ŅĖ®³żČ„
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŅŗĢåæÉŅŌÓĆĄ“±£“ę½šŹōÄʵďĒ(””””)
A£®Ė®”””””””””””””””””” B£®ÅØNaOHČÜŅŗ
C£®ĆŗÓĶ D£®CCl4(¦Ń>1 g”¤cm£3)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠø÷×éÖŠµÄĮ½ÖÖĪļÖŹ×÷ÓĆŹ±£¬·“Ó¦Ģõ¼ž»ņ·“Ó¦ĪļÓĆĮæµÄøı䣬¶ŌÉś³ÉĪļƻӊӰĻģµÄŹĒ(””””)
A£®Na2O2ŗĶH2O B£®NaŗĶO2
C£®CuOŗĶC D£®CŗĶO2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ä³Ń§ÉśÓ¦ÓĆĻĀĶ¼ĖłŹ¾µÄ·½·ØŃŠ¾æĪļÖŹµÄŠŌÖŹ£¬ĘäÖŠĘųĢåAµÄÖ÷ŅŖ³É·ÖŹĒĀČĘų£¬ŌÓÖŹŹĒæÕĘųŗĶĖ®ÕōĘų”£»Ų“šĻĀĮŠĪŹĢā£ŗ
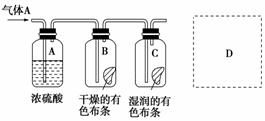
(1)øĆĻīŃŠ¾æ(ŹµŃé)µÄÖ÷ŅŖÄæµÄŹĒ________________________________”£
(2)ÅØĮņĖįµÄ×÷ÓĆŹĒ________£¬ÓėŃŠ¾æÄæµÄÖ±½ÓĻą¹ŲµÄŹµŃéĻÖĻóŹĒ
_____________________________________________________________
_____________________________________________________________ӣ
(3)“ÓĪļÖŹŠŌÖŹµÄ·½ĆęĄ“æ“£¬ÕāŃłµÄŹµŃéÉč¼Ę»¹“ęŌŚŹĀ¹ŹŅž»¼£¬ŌŅņŹĒ
______________________________________________________________
_____________________________________________________________ӣ
ĒėŌŚĶ¼ÖŠD“¦ŅŌĶ¼µÄŠĪŹ½±ķĆ÷æĖ·žŹĀ¹ŹŅž»¼µÄ“ėŹ©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ»Æѧ·“Ó¦ÖŠ£¬ĻõĖįÖ»±ķĻÖŃõ»ÆŠŌµÄŹĒ£Ø £©
A£®3Cu+8HNO3(Ļ”)£½3Cu(NO3)2+2NO”ü+4H2O B£®CuO+2HNO3 (Ļ”)£½Cu(NO3) 2 +H2O
C£®C+4HNO3 (ÅØ)£½CO2 ”ü+4NO2”ü+2H2O D£®3Ag+4HNO3 (Ļ”)£½3 AgNO3 +NO”ü +2H2O
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com