
����Ŀ����״���£�1.68L��ɫ�Ŀ�ȼ������������������ȫȼ�ա���������ͨ����������ʯ��ˮ�У��õ���ɫ��������Ϊ15g������������ʯ������ȼ�ղ������9.3g��
(1)ȼ�ղ�����ˮ������Ϊ_____g��
(2)��ԭ�����ǵ�һ���壬�������ʽΪ_____��������������Ƴɼ���(KOH)ȼ�ϵ�أ���д��������Ӧʽ_____��
(3)��ԭ�����������ֵ����ʵ�������̬����ɵĻ�����д�����ǵķ���ʽ________��(��д������)
(4)��ԭ�����������ֵ����ʵ�����������ɵĻ�������ֻ��һ����������д�����ǵķ���ʽ______��(��д������)
���𰸡�2.7 C2H4 C2H4-12e-+16OH- = 2![]() +10H2O CH4��C3H4��C2H2��C2H6 H2��C4H6��CO��C3H8
+10H2O CH4��C3H4��C2H2��C2H6 H2��C4H6��CO��C3H8
��������
�����л���ȼ��ʱ���л����е�̼ȫ��ת��Ϊ������̼��������̼������������������ȫ��Ӧ�����ɲ�����ˮ�İ�ɫ����̼��ƣ���������̼��Ƶ���������ȼ�����ɶ�����̼����������ʯ�Ҽ�������ȼ�����ɵ�ˮ����������ȼ�����ɵĶ�����̼����ˣ����ص�������ȼ�����ɶ�����̼��ˮ�������ܺͣ��Ӷ����ˮ���������������ɶ�����̼��ˮ�������������ȼ����C��HԪ�ص����ʵ������ٸ��������������ʽ���ݴ˽��
(1)��ȼ�ղ����ж�����̼������Ϊx��
 ��x=6.6g������ʯ�Ҽ�������ȼ�����ɵ�ˮ����������ȼ�����ɵĶ�����̼����ˣ����ص�������ȼ�����ɶ�����̼��ˮ�������ܺ�m(CO2)+m(H2O)=9.3g��m(H2O)=9.3g-6.6g=2.7g��
��x=6.6g������ʯ�Ҽ�������ȼ�����ɵ�ˮ����������ȼ�����ɵĶ�����̼����ˣ����ص�������ȼ�����ɶ�����̼��ˮ�������ܺ�m(CO2)+m(H2O)=9.3g��m(H2O)=9.3g-6.6g=2.7g��
(2)��ɫ��ȼ��������ʵ�����n=![]() = 0.075mol��n(CO2)=
= 0.075mol��n(CO2)=![]() =0.15mol����n(C)=0.15mol��n(H2O)=
=0.15mol����n(C)=0.15mol��n(H2O)=![]() =0.15mol����n(H)=n (H2O)��2=0.3mol����0.075mol�����к���0.15molC��0.3molH������n(����):n(C):n(H)= 0.075mol :0.15mol:0.3mol=1:2:4����1mol�����к���2molC��4molH�����Ը�����ķ���ʽ��C2H4��������������Ƴɼ���(KOH)ȼ�ϵ�أ���������������Ӧ��������ӦʽC2H4-12e-+16OH- = 2
=0.15mol����n(H)=n (H2O)��2=0.3mol����0.075mol�����к���0.15molC��0.3molH������n(����):n(C):n(H)= 0.075mol :0.15mol:0.3mol=1:2:4����1mol�����к���2molC��4molH�����Ը�����ķ���ʽ��C2H4��������������Ƴɼ���(KOH)ȼ�ϵ�أ���������������Ӧ��������ӦʽC2H4-12e-+16OH- = 2![]() +10H2O��
+10H2O��
(3)��Ϊ��һ����ΪC2H4����Ϊ�����ʵ����������������Ļ���������2mol��������У�Ӧ����4molCԭ�ӣ�8molHԭ�ӣ���������̬��������CH4��C3H4��C2H2��C2H6��
��4����Ϊ��һ����ΪC2H4����Ϊ�����ʵ�������������Ļ�������ֻ��һ��������������2mol��������У�Ӧ����4molCԭ�ӡ�8molHԭ�ӣ���������������ǣ�H2��C4H6 ��CO��C3H8�ȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����淴ӦaA��g��+bB��g��cC��g��+dD��g����H������ͼ���жϣ�����������ȷ���ǣ� ��
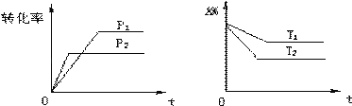
A.p1��p2��a+b��c+d��T1��T2����H��0
B.p1��p2��a+b��c+d��T1��T2����H��0
C.p1��p2��a+b��c+d��T1��T2����H��0
D.���ϴ𰸾�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��Ӧ���ʱ�ͨ����ʵ����вⶨ��Ҳ���������㣬�ش��������⡣
(1)ʵ���ã�1 g CH3OH(l)�������г��ȼ�����ɶ�����̼��Һ̬ˮ�ͷų�22.7kJ����������д���״�ȼ�յ��Ȼ�ѧ����ʽ_______________________________________��
(2)��֪ij��ҵ�����м�����ˮ�����������·�Ӧ��
i.CH4(g)��H2O(g)��CO(g)��3H2(g) ��H1
ii.CO(g)��H2O(g)��CO2(g)��H2(g) ��H2
iii.CH4(g)��C(s)��2H2(g) ��H3
����
IiiΪ��̿��Ӧ�����á�H1�͡�H2�����H3ʱ������Ҫ����_____________��Ӧ�ġ�H��(д��ѧ����ʽ)
(3)�Ȼ���ת��Ϊ�����Ĵ��������£�
CuCl2(s)��CuCl(s)��![]() Cl2(g) ��H1����83 kJ��mol��1
Cl2(g) ��H1����83 kJ��mol��1
CuCl(s)��![]() O2(g)��CuO(s)��
O2(g)��CuO(s)��![]() Cl2(g) ��H2����20 kJ��mol��1
Cl2(g) ��H2����20 kJ��mol��1
CuO(s)��2HCl(g)�� CuCl2(s)��H2O(g) ��H3����121 kJ��mol��1
��4HCl(g)�� O2(g)�� 2Cl2(g)��2H2O(g)����H��___________kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ָ����Ӧ�Ļ�ѧ����ʽ�У���д��ȷ����
A.CH4��Cl2��Ϻ���գ�CH4��Cl2![]() CH2Cl2��H2
CH2Cl2��H2
B.CH2��CH2ͨ��Br2��CCl4��Һ�У�CH2=CH2��Br2��CH3CHBr2
C.CH3CH2OH��Cu��������������O2��Ӧ�� CH3CH2OH��O2![]() CH3COOH��H2O
CH3COOH��H2O
D.CH3COOH��CH3CH2OH ����������Ӧ��CH3COOH��CH3CH2OH![]() CH3COOCH2CH3��H2O
CH3COOCH2CH3��H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CH4��H2��C�������ʵ���Դ����,����ȼ�յ��Ȼ�ѧ����ʽΪ:
��CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H= -890.3 kJ��mol-1
��2H2(g)+O2(g)=2H2O(l) ��H=-571.6 kJ��mol-1
��C(s)+O2(g)=CO2(g) ��H=-393.5 kJ��mol-1
��1������д���һ�ּ���ϸ��,��������øʹ������O2���ö��������������,����ϸ��ʹ1 mol��������CO2������Һ̬ˮ,�ų�������____________(�>������<����=��)890.3 kJ��
��2��������CO2�����ںϳɺϳ���(��Ҫ�ɷ���һ����̼������):CH4+CO2= 2CO+2H2,1 g CH4��ȫ��Ӧ���ͷ�15.46 kJ������,��:

����ͼ(�������ʾ�Ϊ��̬)�ܱ�ʾ�÷�Ӧ�����������仯����__________(����ĸ)��
���������ʵ�����Ϊ1 mol��CH4��CO2����ij�����ܱ�������,��ϵ�ų���������ʱ��ı仯��ͼ��ʾ,��CH4��ת����Ϊ ��

��3��C(s)��H2(g)����Ӧ,����C(s)+2H2(g)=CH4(g)�ķ�Ӧ����ֱ�Ӳ���,��ͨ��������Ӧ�����C(s)+2H2(g)=CH4(g)�ķ�Ӧ�Ȧ�H= ��
��4��Ŀǰ���������������ʵ��о���ȼ���о����ص�,���й��������������ʵ��о������п��е���_____________(����ĸ)��
A��Ѱ�����ʴ���,ʹCO2��H2O��Ӧ����CH4��O2,���ų�����
B������̬̼�ϳ�ΪC60,��C60��Ϊȼ��
C��Ѱ�����ʴ���,����̫����ʹ�����е�CO2�뺣���ɵ�CH4�ϳɺϳ���(CO��H2)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����25��ʱ��H2R�������ε���Һ�У�H2R��HR-��R2-�ֱ�����������ռ�����ʵ�������(��)����ҺpH�仯��ϵ����ͼ��ʾ������������������
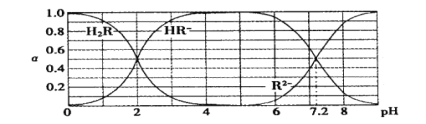
A��H2R�Ƕ�Ԫ���ᣬ��Ka1=1��10-2
B������Һǡ�ó�����ʱ��c( Na + )=2c ( R2- ) + c( HR- )
C��NaHR����Һ��ˮ��������ڵ�������
D����Na2R��NaHR��0.1 mol�Ļ����Һ��pH=7.2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����(H2NNH2)��һ�ָ���ȼ�ϣ��йػ�ѧ��Ӧ�������仯����ͼ��ʾ����֪����1 mol��ѧ�����������(kJ)��N��NΪ942��O=OΪ500��N��NΪ154�������1 mol N��H�����������(kJ)�ǣ�
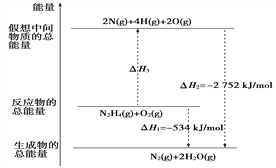
A. 194 B. 391 C. 516 D. 658
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����![]()
A.�����л��е���ϩ��ͨ�����Ը��������Һ��ȥ
B.��Ũ���ᡢŨ����ͱ��ڼ��������¿��Ƶ�������
C.���÷�Һ©�����뱽�뻷����
D.��������������Һ����������Һ����±�����е�±ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������������������ͻ�ѧ���ԣ����������������Ǹ�����ҵ����Ҫԭ�ϡ��ʻ����ᴿ�����漰��������Ӧ����Ϊ
��Ni(s)��4CO(g)![]() Ni(CO)4(g) ��H<0
Ni(CO)4(g) ��H<0
��Ni(CO)4(g)![]() Ni(s)��4CO(g) ��H>0
Ni(s)��4CO(g) ��H>0
���������գ�
��1�����¶Ȳ��������£�Ҫ��߷�Ӧ����Ni(CO)4�IJ��ʣ��ɲ�ȡ�Ĵ�ʩ��____��____��
��2����֪��һ��������2L�ܱ��������Ʊ�Ni(CO)4������(����98.5%���������ʲ���CO��Ӧ)ʣ�������ͷ�Ӧʱ��Ĺ�ϵ��ͼ��ʾ��Ni(CO)4��0��10min��ƽ����Ӧ����Ϊ_____��
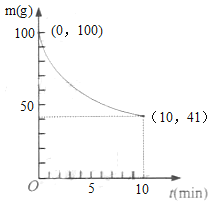
��3������Ӧ�ڴﵽƽ����������������䣬�����¶ȣ����´ﵽƽ��ʱ___��
a��ƽ�ⳣ��K���� b��CO��Ũ�ȼ�С
c��Ni��������С d������[Ni(CO)4]����
��������֪NO2��N2O4�����ת����2NO2(g)![]() N2O4(g) ��H����57kJ��mol��1��һ���¶��£���1molN2O4����һ��ѹ�ܱ������С�
N2O4(g) ��H����57kJ��mol��1��һ���¶��£���1molN2O4����һ��ѹ�ܱ������С�
��1������ʾ��ͼ��ȷ����˵����Ӧ�ﵽƽ��״̬����___��

��2��������ͬ�¶��£�������Ӧ�������Ϊ1L�ĺ����ܱ������н��У�ƽ�ⳣ��____(��������䡱��С��)����Ӧ3s��NO2�����ʵ���Ϊ0.6mol����0��3s�ڵ�ƽ����Ӧ������(N2O4)��___mol��L��1��s��1��
��3���ﵽƽ�������ú�ѹ�������ٳ���0.5molHe����ƽ�⽫__(������ƶ����������ƶ������ƶ���)��
��4���÷�Ӧ���¶ȷֱ�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����___(����ĸ���)��

a.A��C�����������ɫ��A�Cdz
b.A��C����NO2��ת���ʣ�A��C
c.B��C����������ƽ����Է���������B��C
d.��״̬B��״̬A�������ü��ȵķ���
��������ϩ��һ����Ҫ�Ļ���ԭ�ϣ����ɶ���������Ʊ����ش��������⣺
��1��������(C4H10)������1��ϩ(C4H8)���Ȼ�ѧ����ʽ���£�
��֪��
��C4H10(g) ![]() C4H8(g)��H2(g) ��H1
C4H8(g)��H2(g) ��H1
��C4H10(g)��![]() O2(g)=C4H8(g)��H2O(g) ��H2����119kJ��mol��1
O2(g)=C4H8(g)��H2O(g) ��H2����119kJ��mol��1
��H2(g)��![]() O2(g)=H2O(g) ��H3����242kJ��mol��1
O2(g)=H2O(g) ��H3����242kJ��mol��1
��Ӧ�ٵĦ�H1Ϊ____kJ��mol��1��ͼ(a)�Ƿ�Ӧ��ƽ��ת�����뷴Ӧ�¶ȼ�ѹǿ�Ĺ�ϵͼ��x___0.1(����ڡ���С�ڡ�)����ʹ��ϩ��ƽ�������ߣ�Ӧ��ȡ�Ĵ�ʩ��___(����)��
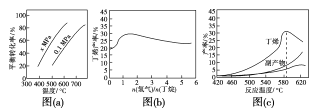
A.�����¶� B.�����¶�
C.����ѹǿ D.����ѹǿ
��2������������Ļ��������һ������ͨ������д����ķ�Ӧ��(�����������ǻ����)���������к��ж�ϩ�����顢�����ȡ�ͼ(b)Ϊ��ϩ�������������n(����)/n(����)�Ĺ�ϵ��ͼ�����߳��������ߺ͵ı仯���ƣ��併�͵�ԭ����__��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com