

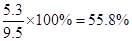 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na2O2�����е��������������ӵ����ʵ���֮��Ϊ1��1 |
| B��9.2 g������Ͷ�뵽��������ˮ�У�������������к���0.4 mol���� |
| C�����ڻ�ѧ��Ӧ�г���ʧȥ���ӱ�������������������Na������ǿ������ |
| D���ơ����ǵ��۵��������������ơ��غϽ��ڳ���ʱ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ˮ��ȷ��ijNa2O��ĩ���Ƿ���Na2O2 |
| B������CO2��ȷ��ijNa2O��ĩ���Ƿ���Na2O2 |
| C���������ڿ����м��ȵķ�����ȥNa2O�е�Na2O2 |
| D����������Na2O2��Na2O�ֱ�ӵ���̪��Һ�У�������Һ��Ϊ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH4��C2H4 | B��CH3CH2OH ��CH3COOH | C��C2H6��HCHO | D��HCHO��HCOOCH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2O | B��CH3COOH | C��CH3CH2OH | D��C6H5OH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������Na2CO3��NaHCO3�ֱ�������ϡH2SO4��Ӧ��NaHCO3������CO2�� |
| B����������Na2CO3��NaHCO3�ֱ���������ͬ�����ᷴӦ��NaHCO3���ĵ������ |
| C����NaHCO3��Һ�е���Ba(OH)2��Һ����������Na2CO3��Һ�м���Ba(OH)2��Һ���ְ�ɫ���� |
| D��Na2CO3��NaHCO3���������ᷴӦ���������������Ʒ�Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com