
��10�֣���ij2 L���ܱ������м���0.1 mol�ⵥ�ʺ�������(W)����943 Kʱ��������ӦW(s)��I2(g)  WI2(g)����I2��ת����Ϊ20%ʱ���ﻯѧƽ��״̬��
WI2(g)����I2��ת����Ϊ20%ʱ���ﻯѧƽ��״̬��
��1����Ӧ�ӿ�ʼ��ƽ��״̬��������ʱ20 s����ƽ����Ӧ����v(WI2)Ϊ________��
��2��943 Kʱ���÷�Ӧ��ƽ�ⳣ��K��________��
��3������ʼʱ������ĵ�Ϊ0.2 mol(������������)��ƽ��ʱ����������������
V(I2)��V(WI2)��________��
��4��������(1)ƽ��״̬�£�ͬʱ����I2(g)��WI2(g)��0.02 mol(������������)����ѧƽ����________(�����Ӧ�����淴Ӧ��)�����ƶ����жϵ�������______________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ�����е�һ��ѧ2011��2012ѧ��߶���ѧ�����л�ѧ���� ���ͣ�022
��ij2 L���ܱ������м���0.1 mol�ⵥ�ʺ�������(W)����943 Kʱ��������ӦW(s)��I2(g)WI2(g)����I2��ת����Ϊ20��ʱ���ﻯѧƽ��״̬��
(1)��Ӧ�ӿ�ʼ��ƽ��״̬��������ʱ20 s����ƽ����Ӧ����v(WI2)Ϊ________��
(2)943 Kʱ���÷�Ӧ��ƽ�ⳣ��K��________��
(3)����ʼʱ������ĵ�Ϊ0.2 mol(������������)��ƽ��ʱ����������������V(I2)��V(WI2)��________��
(4)������(1)ƽ��״̬�£�ͬʱ����I2(g)��WI2(g)��0.02 mol(������������)����ѧƽ����________(�����Ӧ�����淴Ӧ��)�����ƶ����жϵ�������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���ij2 L���ܱ������м���0.1 mol�ⵥ�ʺ�������(W)����943 Kʱ��������ӦW(s)��I2(g) WI2(g)����I2��ת����Ϊ20%ʱ���ﻯѧƽ��״̬��
��1����Ӧ�ӿ�ʼ��ƽ��״̬��������ʱ20 s����ƽ����Ӧ����v(WI2)Ϊ________��
��2��943 Kʱ���÷�Ӧ��ƽ�ⳣ��K��________��
��3������ʼʱ������ĵ�Ϊ0.2 mol(������������)��ƽ��ʱ����������������
V(I2)��V(WI2)��________��
��4��������(1)ƽ��״̬�£�ͬʱ����I2(g)��WI2(g)��0.02 mol(������������)����ѧƽ����________(�����Ӧ�����淴Ӧ��)�����ƶ����жϵ�������______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ�����е�һ��ѧ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��10�֣���ij2 L���ܱ������м���0.1 mol�ⵥ�ʺ�������(W)����943 Kʱ��������ӦW(s)��I2(g) WI2(g)����I2��ת����Ϊ20%ʱ���ﻯѧƽ��״̬��
WI2(g)����I2��ת����Ϊ20%ʱ���ﻯѧƽ��״̬��
��1����Ӧ�ӿ�ʼ��ƽ��״̬��������ʱ20 s����ƽ����Ӧ����v(WI2)Ϊ________��
��2��943 Kʱ���÷�Ӧ��ƽ�ⳣ��K��________��
��3������ʼʱ������ĵ�Ϊ0.2 mol(������������)��ƽ��ʱ����������������
V(I2)��V(WI2)��________��
��4��������(1)ƽ��״̬�£�ͬʱ����I2(g)��WI2(g)��0.02 mol(������������)����ѧƽ����________(�����Ӧ�����淴Ӧ��)�����ƶ����жϵ�������______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������ ���ͣ������
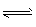 WI2(g)����I2��ת����Ϊ20%ʱ���ﻯѧƽ��״̬��
WI2(g)����I2��ת����Ϊ20%ʱ���ﻯѧƽ��״̬���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com