
| 1.008L |
| 22.4L/mol |
| n |
| V |
| n |
| c |
| ||
| ||
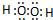 ��
��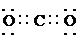 ��
��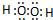 ��
��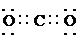 ��
��| 1.008L |
| 22.4L/mol |
| 0.005mol |
| 0.1L |
| 0.2mol |
| 1mol/L |


 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������16�֣����ᱵ��Ψһ�����ı��Σ���ҵ�������ᱵ��Ϊԭ��ͨ���������̷�Ӧ�����Ʊ�п���ף�BaSO4+ZnS���������⡣��𩷯ΪZnSO4•7H2O��
��1�����������й���7����ѧ��Ӧ��������____________������������ԭ��Ӧ��
��2��д���������������C�ĵ���ʽ��____________________��_______________��
��3��д��F��G�Ļ�ѧʽ�� F_____________��G_________________��
��4��д�����л�ѧ��Ӧ����ʽ��
��Ӧ��__________________________________________________________��
��Ӧ��____________________________________________________��
��5��ȡп������16.5g����100mL 1mol/L��H2SO4 ��Һ�У��ų�H2S ����1008mL��������ɱ�״����
�ٲ�����Һ����仯��������Һ������������ʵ���Ũ��Ϊ________mol/L
�ڼ���������Һ��H2S ��Ϊʹп���Ӹպ���ȫ������Ӧ���� 1 mol/L��NaOH��Һ_____mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡ��ϰѧУ��������������������ۺϻ�ѧ���� ���ͣ������
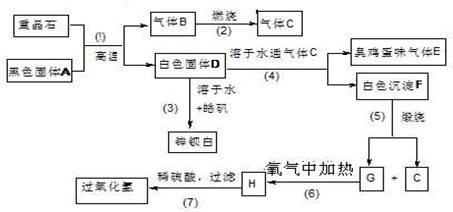
������16�֣����ᱵ��Ψһ�����ı��Σ���ҵ�������ᱵ��Ϊԭ��ͨ���������̷�Ӧ�����Ʊ�п���ף�BaSO4+ZnS���������⡣��𩷯ΪZnSO4?7H2O��
��1�����������й���7����ѧ��Ӧ��������____________������������ԭ��Ӧ��
��2��д���������������C�ĵ���ʽ��____________________��_______________��
��3��д��F��G�Ļ�ѧʽ�� F_____________��G_________________��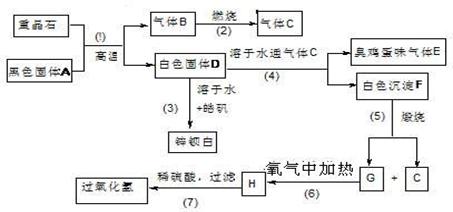
��4��д�����л�ѧ��Ӧ����ʽ��
��Ӧ��__________________________________________________________��
��Ӧ��____________________________________________________��
��5��ȡп������16.5g����100mL 1mol/L��H2SO4��Һ�У� �ų�H2S ����1008mL��������ɱ�״����
�ų�H2S ����1008mL��������ɱ�״����
�ٲ�����Һ����仯��������Һ������������ʵ���Ũ��Ϊ________mol/L
�ڼ���������Һ��H2S ��Ϊʹп���Ӹպ���ȫ������Ӧ���� 1 mol/L��NaOH��Һ_____mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡ��������������������ۺϻ�ѧ���� ���ͣ������
������16�֣����ᱵ��Ψһ�����ı��Σ���ҵ�������ᱵ��Ϊԭ��ͨ���������̷�Ӧ�����Ʊ�п���ף�BaSO4+ZnS���������⡣��𩷯ΪZnSO4•7H2O��
��1�����������й���7����ѧ��Ӧ��������____________������������ԭ��Ӧ��
��2��д���������������C�ĵ���ʽ��____________________��_______________��
��3��д��F��G�Ļ�ѧʽ�� F_____________��G_________________��
��4��д�����л�ѧ��Ӧ����ʽ��
��Ӧ��__________________________________________________________��
��Ӧ��____________________________________________________��
��5��ȡп������16.5g����100mL 1mol/L��H2SO4 ��Һ�У��ų�H2S ����1008mL��������ɱ�״����
�ٲ�����Һ����仯��������Һ������������ʵ���Ũ��Ϊ________mol/L
�ڼ���������Һ��H2S ��Ϊʹп���Ӹպ���ȫ������Ӧ���� 1 mol/L��NaOH��Һ_____mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������16�֣����ᱵ��Ψһ�����ı��Σ���ҵ�������ᱵ��Ϊԭ��ͨ���������̷�Ӧ�����Ʊ�п���ף�BaSO4+ZnS���������⡣��𩷯ΪZnSO4??7H2O��
��1�����������й���7����ѧ��Ӧ��������____________������������ԭ��Ӧ��
��2��д���������������C�ĵ���ʽ��____________________��_______________��
��3��д��F��G�Ļ�ѧʽ�� F_____________��G_________________��

��4��д�����л�ѧ��Ӧ����ʽ��
��Ӧ��__________________________________________________________��
��Ӧ��____________________________________________________��
��5��ȡп������16.5g����100mL 1mol/L��H2SO4 ��Һ�У��ų�H2S ����1008mL��������ɱ�״����
�ٲ�����Һ����仯��������Һ������������ʵ���Ũ��Ϊ________mol/L
�ڼ���������Һ��H2S ��Ϊʹп���Ӹպ���ȫ������Ӧ���� 1 mol/L��NaOH��Һ_____mL
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com