
��֪��2CxHy+(4x+y)CuO![]() 2xCO2+(4x+y)Cu+yH2O
2xCO2+(4x+y)Cu+yH2O
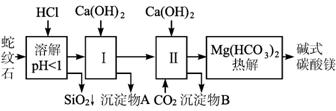
(1)��ֻ�ⶨSO2���壬����ѡ�õ�װ����____________ (��װ����ţ���ͬ)����ֻ�ⶨ��������е�CO2������ѡ�õ�װ����________________________��
(2)����������ֱ�����ͨ���ۢܢ٢ڢݺۢ͢ܢݢڢ٣��Ƿɲ������������������������____________(��ܡ����ܡ�)��
(3)���������������Ϊm g���ֱ�����ͨ���ۢܢ٢ڢݣ�ʵ���װ�ü���m1 g,?��װ������m2 g����ԭ���������CH4����������w(CH4)= ____________��
(4)������������м��黻����ϩ����������(��֪��3CH2==CH2+2MnO4(ϡ)+4H2O![]()
3CH2OH��CH2OH+2KOH+2MnO2��)����ÿ��ԭ�������ȡ����Ϊm g��ֻѡ��װ���еĢۡ��ܡ��ݣ��ܷ�ⶨ���������SO2������������_________ (��ܡ����ܡ�)������ܣ����Ҫд�������Ʒ����ͽ����_____________________________________________��������ܣ��˿ո��
ͼ1-5-20
����:(1)SO2��CO2�����������壬�Կɱ������ա���SO2���н�ǿ��ԭ�ԣ�CO2���������ԣ���ֻ�ⶨSO2ʱ����ѡ��װ�âۡ���ֻ�ⶨCO2��������SO2���پ�����ܱ������գ�����ѡ�õ�װ��Ϊ�ۢ٢ܻ�ۢ٢ݡ�
(2)���徭���ۿɲ��SO2��������ͨ���ܿɲ��CO2�����������ٸ������ͨ���ڣ���ʱ����H2��CH4�����ʵ�����ʾ��Ӧǰ��������������â����ص����ֿɵõ���H2��CH4���ʵ����ķ��̣�����ö��ߵ����ʵ������������������һ�£�˳��ߵ�����Ӱ������������˳���������Ҫ��
(3)��CH4��H2�����ʵ����ֱ�Ϊx��y����
CH4+4CuO![]() 4Cu+CO2+2H2O ������
4Cu+CO2+2H2O ������
1 mol 1 mol 2 mol 64 g
x (x) (2x) (64x)
H2+CuO![]() Cu+H2O ������
Cu+H2O ������
1 mol 1 mol 16 g
y (y) (16y)
����������
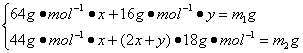
���![]()
����w(CH4)=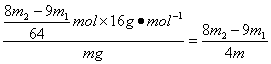
(4)�ܵ�������Ȼ�DzⶨCO2����������ʹ�������ͨ��װ�âۣ���������������Ϊ��ϩ��SO2�������͡���ȡ����������ͨ��װ�âݣ���SO2�����գ���ͨ����ʱ������Ϊ��ϩ�������������ΪSO2����������������SO2������������
��:(1)�� �ۢ٢ܻ�ۢ٢�(��ܻۢ�ۢ�Ҳ��) (2)�� (3)![]()
(4)�� ��ȡm g�����������ͨ��װ�âۣ����װ�â����ӵ���������Ϊa g����ȡm g���������������ͨ��װ�â���ͨ��װ��
�ۣ���������ӵ�����Ϊb g��w(SO2)=![]()



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
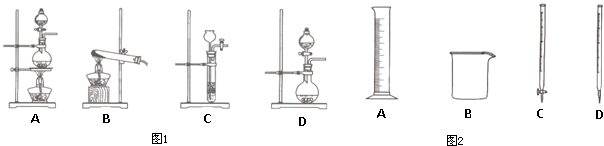
| ��� | ʵ������ | ʵ��ԭ�� | ���巢��װ�� |
| �� | ������ | H2O2��O2 | |
| �� | �ư��� | NH4Cl��NH3 | |
| �� | ������ | HCl��Cl2 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | ʵ������ | ʵ��ԭ�� | ���巢��װ�� |
| �� | ������ | H2O2��O2 | D D |
| �� | �ư��� | NH4Cl��NH3 | B B |
| �� | ������ | HCl��Cl2 | A��D A��D |



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
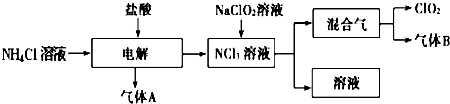
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
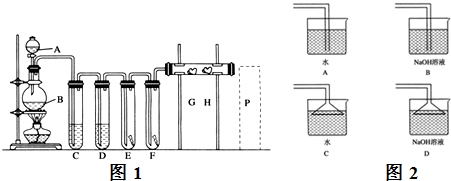
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com