
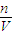 ���ж�ʵ�������n��V��Ӱ�죬�Դ��ж�ʵ����
���ж�ʵ�������n��V��Ӱ�죬�Դ��ж�ʵ����


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�����У�ʮһ���У���һ��ѧ������������ѧ�Ծ� ���ͣ�ʵ����
ʵ������̼���ƾ��壨Na2CO3?10H2O������1.00 mol/L��Na2CO3��Һ240mL���ش��������⣺
��1��������Ҫ����Ϊ��ҩ�ס�������ƽ���ձ�����Ͳ�� ��
�ͽ�ͷ�ιܣ�
��2����ʵ�������̼���ƾ��壨Na2CO3?10H2O�� g��
��3������ƿ�ϱ��п̶��ߡ� �� ��
��4���Է������в�������������Һ��Ũ���к�Ӱ�졣
��������ʱ���Ӷ������ᵼ��������ҺŨ�� ��
������δ�����������ƿȥ������Һ���ᵼ��������ҺŨ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������̼���ƾ�������1.00 mol��L��1��Na2CO3��Һ100 mL���ش��������⣺
(1)������Ҫ����Ϊ��ҩ�ס�__________��__________��__________��__________��__________��__________��
(2)��ʵ�������̼���ƾ���(Na2CO3��10H2O)������Ϊ__________g��
(3)����ƿ�ϱ���__________��__________��������__________��
(4)ijͬѧ�������õ�̼���ƾ���������������ˮ���ձ����ܽ⣬��ȴ��ֱ�ӵ�����ѡ���Ҿ���鲻©ˮ������ƿ�У�ϴ���ձ���������2��3�Σ�ϴ��ҺҲ��������ƿ�У�Ȼ���ˮ����̶���1��2 cm�����ý�ͷ�ιܼ�ˮ���̶��ߣ�Ȼ����Һת�Ƶ��Լ�ƿ�С���ָ�����������е�3������
��______________________________________________________________________��
��______________________________________________________________________��
��______________________________________________________________________��
(5)����������Һ�����������ҺŨ��ƫ�͵�ԭ����__________��
A������̼���ƾ���(Na2CO3��10H2O)��ʱ��̫�������ұ�¶�ڿ�����
B��������ƿ��ת��Һ��ʱ������ƿ����������ˮϴ��
C������ʱ���ӿ̶���
D��ϴ��Һת��������ƿ��ʱ������һ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com