
ijʵ��С���H2O2�ķֽ���������̽����
�±��Ǹ�ʵ��С���о�Ӱ��H2O2�ֽ����ʵ�����ʱ��¼��һ�����ݣ�
��10mL H2O2��ȡ150mL����״���£�O2�����ʱ�䣨s��
| 30%H2O2 | 15%H2O2 | 10%H2O2 | 5%H2O2 | |
| ���� | ��������Ӧ | ��������Ӧ | ��������Ӧ | ��������Ӧ |
| ���� | 360 | 480 | t | 720 |
| MnO2���� | 10 | 25 | 60 | 120 |
��1���ٸ�ʵ��С������Ʒ���ʱ��������Ũ�ȡ�______�� _________ �����ض�H2O2�ֽ����ʵ�Ӱ�졣�������ض�H2O2�ֽ����ʵ�Ӱ��ֱ��ǣ�
______________________________________��
______________________________________��
______________________________________��
���Ʋ�t��s���ķ�ΧΪ______________��
��2����������ͬ���ۼ�״̬��ͬ��MnO2�ֱ����15 mL 5%��H2O2��Һ�У����ô����ǵ�ľ�����ԣ�������£�
| ���� | ���� | �۲��� | ��Ӧ��� ����ʱ�� |
| ��ĩ״ | ��� | ���ҷ�Ӧ�������ǵ�ľ����ȼ | 3.5min |
| ��״ | ��Ӧ���������Ǻ�����ľ��δ��ȼ | 30min |
��д������ʵ���з�����Ӧ�Ļ�ѧ����ʽ__________________ ��
��ʵ���������������Ĵ�Ч����____________________�йء�
��3�������йش�����˵������ȷ����___________������ţ�
A�����������뷴Ӧ����Ӧǰ����������ʺ�����������
B�������ڻ�ѧ��Ӧǰ��ѧ���ʺ�����������
C��������ʹ����Ӧ�����ʷ�����Ӧ
D�������ܸı仯ѧ��Ӧ����
E���κλ�ѧ��Ӧ����Ҫ����
F���������и߶ȵ�ѡ���ԣ���רһ�ԣ�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ʵ��������������ԭ�����͵��� �� ��
A. ��ˮӦ�ܱձ��棬�õ��´�
B. ��FeCl2��Һ�м������۷�ֹ��������
C. ����������ʹ�ù�����������߰���������
D. ʵ�������ű���ʳ��ˮ���ռ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���б仯���������仯����
A��������ˮ��Ϻ��� B������Һ������ۻ��
C��ʯ���͵ķֽ� D�������������Ϻ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ��������������������۷�Ӧʱ��Ϊ�˼�����Ӧ�ٶȣ��Ҳ�Ӱ���������������������������м���������
A��NaHSO4�����壩 B��Na2SO4 ��Һ
C��CuSO4�����壩 D��NaCl�����壩
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ڹ�������������Ӧ��ֻ����һ��һ�ȴ�������
A��CH3CH2CH2CH3 B��CH3CH(CH3)2
C��CH3C(CH3)3 D��(CH3)2CHCH2CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ײ�����ȱλ������(MFe2Ox)(3��x��4��M��ʾMn����Co��Zn��Ni�Ķ�������)�������£�����ʹ��ҵ�����е�SO2��NO2��������ת��Ϊ���ʡ�ת��������ͼ��ʾ�������йظ�ת�����̵�������ȷ����
(����)��
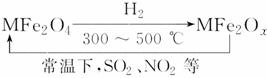
A��MFe2O4�����˻�ԭ��
B��MFe2Ox�����˻�ԭ��
C��SO2��NO2�����˻�ԭ��
D��SO2��NO2�����˷ֽⷴӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й�������������ȷ���� (����)��
�����ܱ������������������ױ���ʴ�����������ڵ�Ѫ�쵰���к�����Ԫ�ء�����λ��Ԫ�����ڱ��е������ڵڢ�B�塡�������������о���ȼ�գ���������ˮ������ȼ�ա�������ǿ���������ᷴӦ�IJ������Fe(NO3)3������ͨ�����Ϸ�Ӧ�Ƶ�FeCl2��Fe(OH)3
A���٢� B���ڢ� C���ڢ� D���ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У��ȿ���Ũ�������ֿ��ù���NaOH������ǣ� ����
A��Cl2 B��SO2 C��O2 D��NH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ҽ�û�ѧ��־����������һ�ֹ��ܸ߷��Ӳ���Ϊ���ҽ�����ά����C�������ֹ��ܸ߷��Ӳ����Ƴ���������߱���Ȼ�߷��Ӳ��ϵij��ߺá����ĺϳɹ���������ʾ��
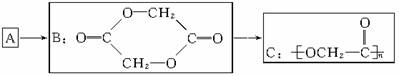
��1��д��A�Ľṹ��ʽ________________________��
��2��д����A��ȡB�Ļ�ѧ����ʽ__________________________________________��
��3��������ҽ�õĸ߷��Ӳ�����߱���Щ���ܣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com