
ij��A 0.2 mol ����������ȫȼ�պ�����CO2��H2O��1.2 mol���Իش�
��1����A�ķ���ʽΪ_____________��
��2����ȡһ��������A��ȫȼ�պ�����CO2��H2O��3 mol������________g��A�μ��˷�Ӧ��ȼ��ʱ���ı�״���µ�����___________L��
��3������A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�����A�Ľṹ��ʽΪ__________________��
��4������A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���4��������A�����еĽṹ���ʽ__________________________________________��
��11�֣���C6H12 ��1�֣� ��42 ��1�֣� 100.8 ��1�֣�
��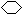 ��1�֣� ��(CH3)3CCH=CH2��CH2=C(CH3)CH(CH3)2��(CH3)2C=C(CH3)2��ÿ��2�֣���
��1�֣� ��(CH3)3CCH=CH2��CH2=C(CH3)CH(CH3)2��(CH3)2C=C(CH3)2��ÿ��2�֣���
���������������1����A 0.2 mol ����������ȫȼ�պ�����CO2��H2O��1.2 mol����A������̼��ԭ�ӵĸ����ֱ���6����12������ѧʽӦ����C6H12��
��2����A��ȫȼ�պ�����CO2��H2O��3 mol�������A�Ļ�ѧʽ��֪���μӷ�Ӧ��A�����ʵ�����0.5mol����A��������0.5mol��84g/mol��42g��
��3����A����ʹ��ˮ��ɫ��˵��������û��̼̼˫��������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣���˵�����ӵ���ԭ��ֻ��һ�࣬����A�ǻ����飬�ṹ��ʽ��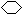 ��
��
��4������A��ʹ��ˮ��ɫ��˵�������Ǻ���̼̼˫���ġ��ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���4�����������A�����еĽṹ���ʽ(CH3)3CCH=CH2��CH2=C(CH3)CH(CH3)2��(CH3)2C=C(CH3)2��
���㣺�����л��ﻯѧʽ���ṹ��ʽ���ж��Լ���ȼ�յ��йؼ���
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����������У��ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ���������������ͷ�ɢ˼ά����������Ĺؼ�����ȷ���ֹ����Žṹ�����ʣ�Ȼ��������������⡢����������ɣ�����������ѧ��������˼ά������Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)
(2)��A��ʹ��ˮ��ɫ�����ڴ�����������H2�ӳɵIJ�������к���4��������A���ܵĽṹ��ʽΪ(��дһ��) __________________________________��
(3)ij�л���ķ���ʽΪCxHyO2����x��ֵ��A�����е�̼ԭ�Ӹ�����ͬ����÷�����y�����ֵΪ__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��A 0.2 mol��O2�г��ȼ��ʱ�����ɻ�����B��C��1.2 mol���Իش�
��1����A�ķ���ʽ________��B��C�ķ���ʽ�ֱ���________��________��
��2����ȡһ��������Aȼ�պ�����B��C��3 mol������________g��A�μ��˷�Ӧ��ȼ��ʱ���ı�״����O2________L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��A 0.2 mol��O2�г��ȼ��ʱ�����ɻ�����B��C��1.2 mol��������������⣺
(1)��A�ķ���ʽ_________��B��C�ķ���ʽ�ֱ���_________��_________��
(2)��ȡһ��������Aȼ�պ�����B��C��3 mol������_________ g��A�μ��˷�Ӧ��ȼ��ʱ���ı�״����O2_________L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������������ѧ�߶��ڶ����¿���ѧ�������Ծ� ���ͣ������
��14�֣�ij��A 0.2 mol ����������ȫȼ�պ�����CO2��H2O��1.2 mol���Իش�
��1����A�ķ���ʽΪ_____________��
��2����ȡһ��������A��ȫȼ�պ�����CO2��H2O��3 mol������________g��A�μ��˷�Ӧ��ȼ��ʱ���ı�״���µ�����___________L��
��3������A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�����A�Ľṹ��ʽΪ__________________��
��4������A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���4��������A�����еĽṹ��ʽΪ_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012��ɽ���ij�ݷ��ʵ����и߶�������ģ����Ի�ѧ�Ծ��������棩 ���ͣ������
��ÿ��2�֣���10�֣�ij��A 0.2 mol ����������ȫȼ�պ�����CO2��H2O��1.2 mol���Իش�
��1����A�ķ���ʽΪ_____________��
��2����ȡһ��������A��ȫȼ�պ�����CO2��H2O��3 mol������________g��A�μ��˷�Ӧ��ȼ��ʱ���ı�״���µ�����___________L��
��3������A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ
��һ�֣�����A�Ľṹ��ʽΪ__________________��
��4������A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���
4��������A�����еĽṹ��ʽΪ______________��д��һ�ּ��ɣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com