
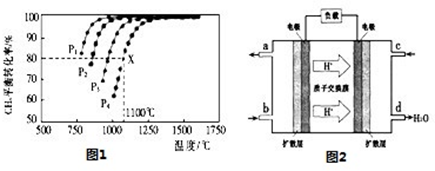
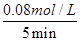 ��0.016mol/(L?min)����������֮�ȵ��ڻ�ѧ������֮�ȿ�֪��v(CO)��2v v(CH4)��2��0.016mol/(L?min)��0.032mol/(L?min)��
��0.016mol/(L?min)����������֮�ȵ��ڻ�ѧ������֮�ȿ�֪��v(CO)��2v v(CH4)��2��0.016mol/(L?min)��0.032mol/(L?min)��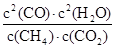 ��
��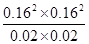 ��1.64
��1.64

 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3��g������Ӧ���̵������仯��ͼ��ʾ����֪N2��g����H2��g����Ӧ����17 g NH3��g�����ų�46.1 kJ��������
2NH3��g������Ӧ���̵������仯��ͼ��ʾ����֪N2��g����H2��g����Ӧ����17 g NH3��g�����ų�46.1 kJ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
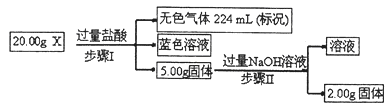
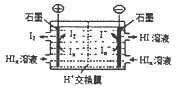
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3 (g) ��H =" -92.4" kJ/mol
2NH3 (g) ��H =" -92.4" kJ/mol
| ��Ӧ | �����̵� | ��ҵ�̵� | ||||
| �¶�/�� | 27 | 2000 | 25 | 350 | 400 | 450 |
| K | 3.84��10-31 | 0.1 | 5��108 | 1.847 | 0.507 | 0.152 |
 2NH3 (g)��ü�������H2��ת����Ϊ40%��
2NH3 (g)��ü�������H2��ת����Ϊ40%��| | N2 | H2 | NH3 |
| �� | 1 | 3 | 0 |
| �� | 0.5 | 1.5 | 1 |
| �� | 0 | 0 | 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������

 CO2(g)��H2(g)���õ������������ݣ�
CO2(g)��H2(g)���õ������������ݣ�| ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | |
| H2O | CO | CO2 | |||
| 1 | 650 | 2 | 4 | 1.6 | 5 |
| 2 | 900 | 1 | 2 | 0.4 | 3 |
| 3 | 900 | 1 | 2 | 0.4 | 1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������

 ��
�� ����ԭ
����ԭ �ķ���Ҳ�������������������Ⱦ�����磺
�ķ���Ҳ�������������������Ⱦ�����磺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CO(NH2)2(l)��H2O(g)�ںϳ����н��С���ͼ�Т���������Ϊ�ϳ����а���ͬ��̼�� [n(NH3)/n(CO2)]��ˮ̼��[n(H2O)/n(CO2)]Ͷ��ʱ������̼ת���ʵ������
CO(NH2)2(l)��H2O(g)�ںϳ����н��С���ͼ�Т���������Ϊ�ϳ����а���ͬ��̼�� [n(NH3)/n(CO2)]��ˮ̼��[n(H2O)/n(CO2)]Ͷ��ʱ������̼ת���ʵ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| | �� | �� | Na2CO3 | ���ʯ | ʯī |
| �۵㣨�棩 | 63.65 | 97.8 | 851 | 3550 | 3850 |
| �е㣨�棩 | 774 | 882.9 | 1850���ֽ����CO2�� | ---- | 4250 |
 2 Na2CO3��l��+ C(s�����ʯ) ��H=��1080.9kJ/mol
2 Na2CO3��l��+ C(s�����ʯ) ��H=��1080.9kJ/mol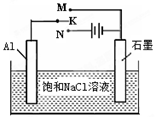
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��12��H3+5��H2-2��H1 | B��2��H1-5��H2-12��H3 |
| C��12��H3-5��H2 -2��H1 | D����H1-5��H2-12��H3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com