
N2�����������;�㷺��ij��ѧ��ȤС��Ϊ̽����ʵ�����Ʊ���Ϊ����N2�ķ��������������������������뽻�������ۡ�
[��������]N2���Ʒ����������ַ�����
����1������NaNO2��NH4Cl��Ũ��Һ�Ƶ�N2��
����2�����������£���NH3��ԭCuO���Ƶ�N2��ͬʱ��û���ͭ�ۡ�
����3������������ͨ�����ȵ�ͭ�ۻ�ýϴ���N2��
[ʵ����]��ʵ���ҳ�������(ҩƷ)����ƵIJ���װ������ͼ(���ּгֺͼ�������δ����)��
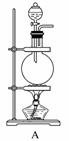
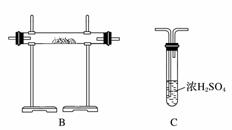
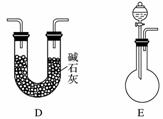
[��������]
(1)���Է���1�Ƶ�N2��Ӧѡ��ķ���װ����_____________________________��
(2)��������2�Ƶø��������N2������Ҫ��NH3����ʯ�Һ�Ũ��ˮ��ԭ�ϣ���������װ�ð����������ҵ�����˳����______________(����ĸ)�����N2�ķ�Ӧԭ����________________________________________________________________________(д��Ӧ����ʽ)��
(3)������ˮ���ռ�N2�����л����ˮ��������Ҳ�������ſ���������ԭ����________________________________________________________________________��
��������ռ�������________________________________________________________________________��
(4)�������������У��Ƶõ�N2����������________����N2����Ҫ�����ϸ������£����˽��齫����2�ͷ���3���ʹ�ã�����Ϊ���ŵ���________________________________________________________________________��
�𰸡�(1)A
(2)E��D��B��C��2NH3��3CuO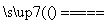 3Cu��N2��3H2O
3Cu��N2��3H2O
(3)N2����Է�������28�������ƽ����Է����������(�������ܶȴ�С�Ƚϻش�����Ҳ��)���������ռ�(����������Ҳ��)
(4)����3��CuO��Cu��ѭ�����ã���ʡҩƷ
������(1)���÷�Ӧ�����ص��뷴Ӧ���״̬�Լ���Ӧ�����йأ�����1Ϊ��������Һ̬���ʣ����ϴ�������ֻ��Aװ�á�(2)����2Ϊ���������ķ�Ӧ����Ӧ��Ӧѡ��B����Ҫ��ͨ��İ��������Ҵ���������Ũ��ˮ����ʯ�ҷ�Ӧ��ȡ����Ϊ��Һ�����ȵ�װ�ã�ѡ��E����ͬʱ��ˮ�������ɣ�������ü�ʯ���������Ӧ��Ϻ������ˮ������û�в��뷴Ӧ�İ����뵪��һͬ����������Ũ����ȿ��Գ�ȥˮ���ֿ��Գ�ȥ������(3)�������ռ�������Ҫ�����������ܶȵIJ��������ÿ���ֱ���ſ��ڲ�������װ�����ռ�������(4)����3�����˳�ȥ�����е��������õ�����ԭ���������г��������͵�����ж�����̼��ϡ�����壬��˲�������


 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ϩ�Ľṹ�ɱ�ʾΪ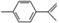 �����й����������ʵ�˵������ȷ����(����)
�����й����������ʵ�˵������ȷ����(����)
A���뱽�Ľṹ���ƣ�����Ҳ����
B����ʹ������Ȼ�̼��Һ��ɫ
C������ȡ����Ӧ���ѷ����ӳɷ�Ӧ
D�������ʼ�������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
CuSO4��Һ��K2C2O4��Һ�������һ����ɫ����ˮ����KxCuy(C2O4)z·nH2O��ͨ������ʵ��ȷ���þ������ɡ�(��֪��MnO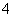 �����������£������ֽܷ�ΪO2��ͬʱ����Mn2����)
�����������£������ֽܷ�ΪO2��ͬʱ����Mn2����)
����a����ȡ0.672 0 g��Ʒ��������ƿ����������2 mol·L��1ϡ���ᣬ��ʹ��Ʒ�ܽ⡣�ټ���30 mLˮ���ȣ���0.200 0 mol·L��1 KMnO4��Һ�ζ����յ㣬����8.00 mL KMnO4��Һ���йط�Ӧ��2MnO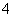 ��5C2O
��5C2O ��16H��===2Mn2����8H2O��10CO2����
��16H��===2Mn2����8H2O��10CO2����
����b�����Ž���Һ��ּ��ȡ���ȴ����pH�����������KI���壬��Һ��Ϊ��ɫ��������ɫ����CuI����0.250 0 mol·L��1 Na2S2O3����Һ�ζ����յ㣬����8.00 mL Na2S2O3��Һ���ζ�ʱ��ӦΪI2��2S2O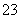 ===2I����S4O
===2I����S4O ��
��
(1)����b�����ɰ�ɫ���������ӷ���ʽ��________________________________________________________________________��
(2)����b�С�����Һ��ּ��ȡ���Ŀ����________________________________________________________________________��
(3)���������ȷ����Ʒ��ɵļ�����̡�
�ټ�����Ʒ��n(C2O )(д���������)
)(��������)
________________________________________________________________________��
�ڼ�����Ʒ��n(Cu2��)(д���������)
________________________________________________________________________��
�۸���________ԭ�������n(K��)��������________ԭ�����n(H2O)��
�ܸ���Ʒ����Ļ�ѧʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�⻯��ͭ(CuH)��һ�ֲ��ȶ����ʣ�����������ȼ�գ�Ҳ�����ᷴӦ����CuSO4��Һ�͡�ij���ʡ���40��50 ��ʱ��Ӧ�ɲ������������й������д������(����)
A����ij���ʡ����л�ԭ��
B��CuH�����ᷴӦ�����ܲ���H2
C��CuH��������ϡ���ᷴӦ��CuH��3H����NO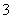 ===Cu2����NO����2H2O
===Cu2����NO����2H2O
D��CuH��������ȼ�գ�CuH��Cl2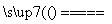 CuCl��HCl
CuCl��HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ƿ���Ϊ���ȼ�����֪25.0 mL 0.100 mol·L��1 Na2S2O3��Һǡ�ð�224 mL(��״����)Cl2��ȫת��ΪCl������S2O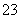 ��ת��Ϊ(����)
��ת��Ϊ(����)
A��S2�� B��S C��SO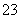 D��SO
D��SO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧʵ���Ҳ����ķ�Һ�к���Fe3����Cu2����Ba2����Cl���������ӣ���������з����Է�Һ���д������Ի��ս������Ʊ��Ȼ������Ȼ������塣

(1)����1�к��еĽ���������__________��
(2)����ʱ����H2O2��Һ������Ӧ�����ӷ���ʽΪ
________________________________________________________________________��
(3)���������У�������Ϊ�Լ�X����__________(����ĸ)��
A��BaCl2 B��BaCO3
C��NaOH D��Ba(OH)2
(4)�������2ϴ���Ƿ���ȫ�ķ�����__________��
(5)�Ʊ��Ȼ�������������豣�������������Ŀ����__________��
(6)�ɹ���2�õ�����Һ�Ʊ�BaCl2��ʵ���������Ϊ__________����ȴ�ᾧ��__________��ϴ�ӡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ƿ������ڸ��Ƶر�ˮ�ʣ��������ؽ������ӷ�ˮ�������ೱ��Ҳ������Ӧ�������ȡ���ҵ�������������Ƶ���Ҫ�������£�
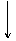
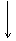 CaCl2���塡30%��H2O2
CaCl2���塡30%��H2O2
����������





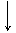
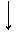






������ ����������������������������
������ NH3 ����Ʒ������ ���������� ����Ʒ
��֪CaO2·8H2O�ʰ�ɫ������ˮ��������350 �����ҿ�ʼ�ֽ�ų�������
(1)������������ȡCaO2·8H2O�Ļ�ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
(2)���顰ˮϴ���Ƿ�ϸ�ķ�����________��
(3)����ʱ���ñ�ˮ�����¶���0 �����ң������ԭ����(д������)��
��________________________________________________________________________��
��________________________________________________________________________��
(4)�ⶨ��Ʒ��CaO2������ʵ�鲽�裺
��һ����ȷ��ȡa g��Ʒ��������ƿ�У�������������ˮ������b g KI���壬�ٵ�������2 mol·L��1��H2SO4��Һ����ַ�Ӧ��
�ڶ�������������ƿ�м��뼸�ε�����Һ��
����������μ���Ũ��Ϊc mol·L��1��Na2S2O3��Һ����Ӧ��ȫ������Na2S2O3��ҺV mL��
(��֪��I2��2S2O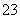 ===2I����S4O
===2I����S4O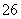 )
)
��CaO2����������Ϊ____________(����ĸ��ʾ)��
��ijͬѧ��һ���͵ڶ����IJ������ܹ淶������������̫����������õ�CaO2��������������________(�����Ӱ�족����ƫ�͡���ƫ�ߡ�)��ԭ����________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̼����ĺ���Ӱ��������ܣ�̼��������һ�ֲⶨ�����ǽ�������̼����ת��Ϊ���壬���ò�̼������װ�ý��вⶨ��
(1)����װ��A���ڸ����½�x�˸�����̼����ת��ΪCO2��SO2��
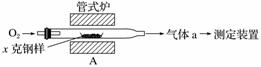
������a�ijɷ���________________��
��������������FeS��ʽ���ڣ�A�з�Ӧ��3FeS��5O2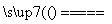
1________��3________��
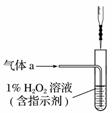
(2)������aͨ�����װ����(����ͼ)�����õζ����ⶨ��ĺ�����
��H2O2����SO2�Ļ�ѧ����ʽ
________________________________________________________________________
________________________________________________________________________��
����NaOH��Һ�ζ����ɵ�H2SO4������z mL NaOH��Һ��������1 mL NaOH��Һ�൱���������Ϊy�ˣ���ø������������������________��
(3)������aͨ���̼װ����(����ͼ)�������������ⶨ̼�ĺ�����
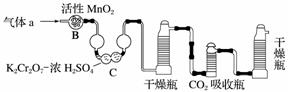
������aͨ��B��C��Ŀ����__________________________________________��
�ڼ��������̼������������Ӧ������������________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
PbO2����PbO�����������Һ��Ӧ�Ƶã���Ӧ�����ӷ���ʽΪ________________________________��PbO2Ҳ����ͨ��ʯīΪ�缫��Pb(NO3)2��Cu(NO3)2�Ļ����ҺΪ���Һ�����ȡ�����������ĵ缫��ӦʽΪ__________________________�������Ϲ۲쵽��������________________�������Һ�в�����Cu(NO3)2�����������ĵ缫��ӦʽΪ________________________������������Ҫȱ����________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com