
��1����8�֣�ij��Ӧ��ϵ�е�������NaOH��Au2O3��Na2S4O6��Au2O��H2O��Na2S2O3��Au2O3Ϊ��Ӧ��ش����в���
| A��NaOH������Ӧ������ |
| B��Na2S4O6��������������ԭ�������������ԭ��� |
| C���������ͻ�ԭ�������ʵ���֮���� |
| D��0.25mol Au2O3������Ӧ��ת�Ƶ������� |
����11�֣���1����8�֣�
A��NaOH ������
B��Na2S4O6�� ��������
C���������ͻ�ԭ�������ʵ���֮���� 1��4
D��0.25molAu2O3������Ӧ��ת�Ƶ������� 6.02��1023
��2����3�֣� 2MnO4-+5SO2+2H2O==2Mn2++5SO42-+4H+
����Au2O3��4Na2S2O3��2H2O = 2Na2S4O6��Au2O��4NaOH���ɷ���ʽ��֪��1molAu2O3ת��4mol���ӡ�
��A��NaOH ������
B��Na2S4O6�� ��������
C���������ͻ�ԭ�������ʵ���֮���� 1��4
D��0.25mol Au2O3������Ӧ��ת�Ƶ�������6.02��1023
��ע����ƽʱ��H������д�ڷ�Ӧ���У�Ӧ���õ����غ���ƽ����������ԭ����ϵ�������õ���غ㣬�ڷ���ʽ���������в���H�� ,2MnO4-+5SO2+2H2O==2Mn2++5SO42-+4H+



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����8�֣�ij��Ӧ��ϵ�е�������NaOH��Au2O3��Na2S4O6��Au2O��H2O��Na2S2O3��Au2O3Ϊ��Ӧ��ش����в���
A��NaOH�� ����Ӧ������
B��Na2S4O6�� ������������ԭ�������������ԭ���
C���������ͻ�ԭ�������ʵ���֮����
D��0.25mol Au2O3������Ӧ��ת�Ƶ�������
��2����3�֣�������������MnO4-��SO2������SO42-��ͬʱ����ԭ��Mn2+.д����Ӧ�����ӷ���ʽ����ƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����������������ѧ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��1����8�֣�ij��Ӧ��ϵ�е�������NaOH��Au2O3��Na2S4O6��Au2O��H2O��Na2S2O3��Au2O3Ϊ��Ӧ��ش����в���
| A��NaOH������Ӧ������ |
| B��Na2S4O6��������������ԭ�������������ԭ��� |
| C���������ͻ�ԭ�������ʵ���֮���� |
D��0.25mol Au2O3������Ӧ��ת�Ƶ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����������������ѧ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��1����8�֣�ij��Ӧ��ϵ�е�������NaOH��Au2O3��Na2S4O6��Au2O��H2O��Na2S2O3��Au2O3Ϊ��Ӧ��ش����в���
A��NaOH�� ����Ӧ������
B��Na2S4O6�� ������������ԭ�������������ԭ���
C���������ͻ�ԭ�������ʵ���֮����
D��0.25mol Au2O3������Ӧ��ת�Ƶ�������
��2����3�֣�������������MnO4-��SO2������SO42-��ͬʱ����ԭ��Mn2+.д����Ӧ�����ӷ���ʽ����ƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������������и���10���¿������ۣ���ѧ���� ���ͣ������
���⺬��С�⣬��14�֡�
��1����8�֣�ij��Ӧ��ϵ�е������У�NaOH��Au2O3��Na2S4O6��Na2S2O3��Au2O��H2O��
�ٽ�Au2O3֮������ʷֱ��������¿ո��ڣ���ƽ��������ת�Ƶķ������Ŀ��
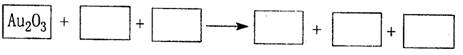
�ڷ�Ӧ�У�����ԭ��Ԫ���� ����ԭ���� ��
�۷�֯��ҵ�г���������Ư����Na2S2O3����ΪƯ��ƥ�ġ����ȼ�����Na2S2O3��Cl2��Ӧ�IJ�����H2SO4��NaCl��HC1������ԭ�������������ʵ���֮��Ϊ
��
��2����6�֣���Ҫ��д����ˮ�����������ԭ��Ӧ�Ļ�ѧ����ʽ
��ˮ����������
��ˮ�ǻ�ԭ����
��ˮ�Ȳ���������Ҳ���ǻ�ԭ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com