
�����仯���������������о�����Ҫ�����á�
��1������Ԫ�����ڱ��е�λ���� ��
��2����֪�縺�Ե���ֵ��ʾԭ�ӶԵ���������������Դ�С�������Ǽ���ԭ�ӵĵ縺����ֵ��
| Ԫ�� | �� | þ | �� | �� |
| �縺�� | 0.9 | 1.2 | �� | 1.8 |
��14�֣�ÿ��2�֣�
��1����3���ڵڢ�A��
��2���ٵ縺����ֵԽ��Ԫ�صĽ�����Խ��
�� 1.2���֣�1.8
�� c
��3��4Al(s)�� 3MnO2(s)��3Mn(s)��2Al2O3(s) ��H���C1789 kJ/mol
��4����
������������Ӧ2O2-�C 4e-��O2����C��O2��CO2��ʯī���ڱ�������Ҫ���ϲ���
���������������1��AlΪ13��Ԫ�أ�λ�ڵ�3���ڵڢ�A�塣
��2���ٸ��ݵ縺�Եı仯���ɣ����ĵ縺�Խ���Mg��Si֮�䡣
�ڵ縺����ֵԽ��ԭ�ӶԵ�����������Խ��ʧ����Խ�ѣ����Խ�����Խ����
��a����������縺���أ�����b�������ʵ���Ũ�ȵ�Al2(SO4)3��MgSO4��Һ��SO42?Ũ�Ȳ�ͬ������c���ӹ���NaOH��Һ��AlCl3��Һ�ȳ��ֳ������ܽ⣬˵��Al(OH)3�����ԣ�����������Mg����ȷ��
��3������д����ѧ����ʽ��ע��״̬��Ȼ����ݸ�˹�������ʱ䣺?H=?H1�C3?H2=" �C1789" kJ?mol?1������д���Ȼ�ѧ����ʽ��
��4��Al3+�������õ�������Al������O2?�õ�������O2���ڽϸ��¶�����ʯī��Ӧ����CO2��ʯī��ر����ģ�������Ҫ���ϲ��䡣
���㣺 ���⿼��Ԫ�����������������ڱ����Ȼ�ѧ����ʽ����д������ԭ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)���û�ѧ��Ӧ�����Ʊ��������ʡ�ʵ������ͭ�Ʊ�NO2�����ӷ���ʽΪ_____________ ______��
��2����ҵ�ϣ���ͭ����Ҫ�ɷ�CuFeS2������ȡͭ����Ҫԭ�ϣ��ɲ��û�������������ͭ���ù��յ��м���̻ᷢ����Ӧ��Cu2S��2Cu2O===6Cu+SO2�����÷�Ӧ�л�ԭ��Ϊ_______ (�ѧʽ)��ÿ����1mol Cu����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ___________��
��ͭ��ұ��ͭ������¯������Fe2O3��FeO��SiO2��Al2O3�����Ʊ�Fe2O3������Ϊ��
����ϡ�����ȡ¯�������ˡ� ����Һ���������ټ������NaOH��Һ�����ˣ�������ϴ�ӡ�������յ�Fe2O3�� ��������Ϣ�ش��������⣺
a��ͨ�������ڣ�¯���е�Al2O3����� ��д���ӣ���
b��ѡ���ṩ���Լ������ʵ����֤¯���к���FeO��
�ṩ���Լ���ϡ���� ϡ���� KSCN��Һ ����KMnO4��Һ NaOH��Һ ��ˮ
��ѡ�Լ�Ϊ ��
֤��¯���к���FeO��ʵ������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������������õķ���ʴ�ԣ��ڹ�����ҵ���зdz���Ҫ�����ã��������ȷ�Ӧ��ɸֹ�ĺ��ӷdz�����Ѹ�١����������գ�
��1��������©���н����ȼ���Ͼ��Ⱥ��������ȷ�Ӧ�IJ����ǣ� ��
��2��������ͬ���ڡ��ؿ���衢���ĺ����� �����,����=����SiO2�ǹ����β�����Na2CaSi6O14����Ҫ�ɷ֣�Na2CaSi6O14Ҳ��д��Na2O��CaO��6SiO2���Ƴ�ʯ��NaAlSi3O8������������ʽ ����ʯ���������Σ���ͬ�ʯ����ԭ�ӵ����ʵ���������ͬ���ɴ˿���֪�Ƴ�ʯ�Ļ�ѧʽΪ ��
��3��ij���Ͻ���Al��Si��Cu��Mg��ɡ��ٳ�ȡ100g�����Ͻ���Ʒ���ֳɵ�������A��B���ݡ���A�ݼ�������NaOH��Һ��B�ݼ���������ϡ���ᡣ�ڴ����ݷ�Ӧ�ﶼ��ַ�Ӧ֮�Ƶ������������1.60g���ռ��õ������������������2240mL(��״����)������Ʒ��Si��Mg�����ʵ����ֱ��� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ��ͨ������������Ҫ�ɷ�ΪAl2O3��Ϊԭ���Ʊ���ˮ�Ȼ�����2Al2O3+6Cl2 4AlCl3+3O2 ������ش��������⣺
4AlCl3+3O2 ������ش��������⣺
��1��������Ӧ�漰��Ԫ���У��������2��δ�ɶԵ��ӵ�Ԫ����__________�������ӵ����Ӱ뾶��С��Ԫ�أ���ԭ�Ӻ�����_____�ֲ�ͬ�ܼ��ĵ��ӡ�
��2����֪Ԫ�����ڱ��У���(31Ga)����Ԫ��ͬһ���壬д��Ga�����������Ų�ʽ��______
��3����(Ga)��������Ȼͬλ�أ�һ����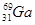 ����ԭ������Ȼͬλ��ԭ������ռ�İٷֱ�Ϊ60%��ʵ�����廯��(GaBr3)��Ħ������Ϊ309.8 g/mol�����ɴ���֪�ص���һ��ͬλ����_________��
����ԭ������Ȼͬλ��ԭ������ռ�İٷֱ�Ϊ60%��ʵ�����廯��(GaBr3)��Ħ������Ϊ309.8 g/mol�����ɴ���֪�ص���һ��ͬλ����_________��
��4��Ϊ�ٽ���Ӧ�Ľ��У�ʵ������������뽹̿����ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����FeCl3��Һ�м���a g��ͭ�ۣ�����ʹ֮ȫ���ܽ⣬��Һ��һ���е���������_________�������е��������� ��������Ӧ�����ӷ���ʽΪ ���������м��� b g���ۣ���ַ�Ӧ����˵�����c g�����ܷ�����Ӧ�����ӷ���ʽΪ ������֪ a��b��c���� c��������_________��
��2������Na2SO3��Һ��ϡH2SO4���밴Ҫ������±���
| ���� | ���� | ������ |
| 1 | �üס�����֧�Թֱܷ�ȡ������Һ�������� | |
| 2 | �ý�ͷ�ι�����Թ�����εμ�BaCl2��Һ�������� | |
| 3 | �� | ������� ������ �� ������� ������ �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
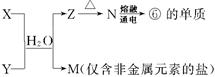
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã�
�ش��������⣺
| �� ���� | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | �� | �� | �� | | | �� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������Li/FeS2��ص������������ʣ�����ˮ�ȷ��ϳɡ�FeSO4��Na2S2O3��S��H2O��200��������Ӧ24Сʱ�����������Ե����ʵ�����Ӧ����������CS2��H2Oϴ�ӡ����P������õ���
��1���ϳ�FeS2���ӷ���ʽΪ ��
��2����ˮϴ��ʱ�����֤��S042-�������� ��
��3����֪1.20gFeS2��O2����ȫȼ������Fe2O3��SO2����ų�8.52kJ������FeS2ȼ�շ�Ӧ���Ȼ�ѧ����ʽΪ�� ��
��4��ȡ�����Ƶõ���������1.1200g (�ٶ�ֻ��FeSһ������)�����������������г�ּ��ȣ�����0.8000g����ɫ���壬�������������FeS2����������(д���������)��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��14�֣���ѧ����Cl2��FeCl2��KSCN�����Һ�ķ�Ӧ����ʵ��̽����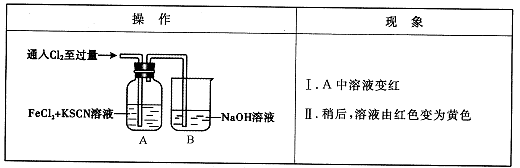
��1��B�з�Ӧ�����ӷ���ʽ��____��
��2��A����Һ����ԭ����____��
��3��Ϊ��̽������II��ԭ��ͬѧ��������ʵ�顣
��ȡA�л�ɫ��Һ���Թ��У�����NaOH��Һ���к��ɫ�������ɣ�����Һ��һ������ ��
��ȡA�л�ɫ��Һ���Թ��У����������KSCN��Һ�����յõ���ɫ��Һ��
��ͬѧ��ʵ��֤������������ԭ����SCN����Cl2�����˷�Ӧ��
��4����ͬѧ����SCN�����ܱ�Cl2�����ˣ����ֽ����������о���
������ʾ��SCN���ĵ���ʽΪ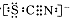
�ټ�ͬѧ��ΪSCN����̼Ԫ��û�б������������� ��
��ȡA�л�ɫ��Һ���Թ��У������������ữ��BaCl2��Һ��������ɫ�������ɴ�֤��SCN���б�������Ԫ���� ��
��ͨ��ʵ��֤����SCN���е�Ԫ��ת��ΪNO3-������ʵ�鷽����____��
����SCN����Cl2��Ӧ����1 mol CO2����ת�Ƶ��ӵ����ʵ����� mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����þ�������ۡ�ͭƬ����������������������ؼ��������õ�ʵ����Ʒ����������������ҩƷ��������Ƽ�ʵ����֤�������������ʵĻ�ԭ��ǿ������������Ƶ�ʵ�鷽�����ش��������⣺
��1��ʵ��ԭ�������û�ѧ����ʽ��ʾ�� ��ʵ��������Ļ�ѧ����Ϊ ��
��2������ʵ��IJ�������Ϊ�� ��
��3��ʵ��̽����ȡ������Ӧ���õ��Ĺ������������������ϡH2SO4���μ�KSCN��Һ���������� �����ܻ��˵��������������Fe2O3����˵������ �����������ӷ���ʽ˵������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com