
��15�֣�W��M��X��Y��Z��Q��ԭ��������������Ķ�����Ԫ�أ�X��Y�ǽ���Ԫ�أ�X����ɫ�ʻ�ɫ������Ԫ�غ˵����֮��Ϊ71��W��Q������������ͬ��Q�ĺ˵������W��2����Z�ĵ��ʺ��������Ϊԭ�Ӿ��塣��ҵ��һ��ͨ�����������ķ������Y�ĵ��ʡ���ش��������⣺
(1)Y�����ӽṹʾ��ͼ____________��MԪ����Ԫ�����ڱ��е�λ��Ϊ______________��
(2)д������ʽ����������ָ��������ѧ�������ͣ�W�ĵ���______________�� ����
X��W��ԭ�Ӹ�����2:1�γɵĻ�����_____________�� ����
(3)W��M��X��Y��Z��Qԭ�Ӱ뾶��С�����˳��Ϊ__________________(��Ԫ�ط��ű�ʾ)��
(4)W��M��Q�γɵ��⻯����ȶ���_______________________(���⻯�����ʽ��ʾ)��ZԪ���⻯��ķ��ӿռ乹��___________________��
(5)X��Y��Q������������Ӧ��ˮ����֮���������ɷ�Ӧ��д����Ӧ�����ӷ���ʽ��
________________________��________________________��______________��
��1��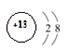 �ڶ����ڵڢ�A��
�ڶ����ڵڢ�A��
��2�� �����ۼ�����
�����ۼ�����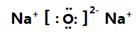 �����Ӽ���
�����Ӽ���
��3��_r(Na)>r(Al)>r(Si)>r(S)>r(O)>r(F) ��4��_HF>H2O>H2S_��_��������_
��5��_H++OH-=H2O_��__3H++Al(OH)3=Al3++3H20__��_Al(OH)3+OH-=AlO2-+2H2O
��������
���������Q��W��X��Y��Z��ԭ��������������Ķ�����Ԫ�أ�X��Y�ǽ���Ԫ�أ�X����ɫ�ʻ�ɫ����XΪ��Ԫ�أ���ҵ��һ��ͨ�����������ķ������Y�ĵ��ʣ���YΪ��Ԫ�أ�W��Z������������ͬ������Ϊͬ���壬Z�ĺ˵������W��2����ԭ������Z����W����WΪ��Ԫ�أ�ZΪ��Ԫ�أ�����Ԫ������������֮��Ϊ20����Q������������Ϊ20-6-1-3-6=4��ԭ������QС����Ԫ�أ���QΪ̼Ԫ�أ����Ԫ�������ɼ��������ʻش�
���㣺ԭ�ӽṹ��Ԫ�������ʵĹ�ϵ
����������Ԫ��λ�ýṹ���ʵĹ�ϵ��Ӧ�ã��Ѷ��еȣ�Ԫ���ƶ��ǹؼ���ѧϰ��ע����ػ���֪ʶ�Ļ��ۡ�


 ȫ��������ϵ�д�
ȫ��������ϵ�д� һ��һ����ʱ���ϵ�д�
һ��һ����ʱ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣�W��M��X��Y��Z��Q��ԭ��������������Ķ�����Ԫ�أ�X��Y�ǽ���Ԫ�أ�X����ɫ�ʻ�ɫ������Ԫ�غ˵����֮��Ϊ71��W��Q������������ͬ��Q�ĺ˵������W��2����Z�ĵ��ʺ��������Ϊԭ�Ӿ��塣��ҵ��һ��ͨ�����������ķ������Y�ĵ��ʡ���ش��������⣺
��1��Y�����ӽṹʾ��ͼ_____________;MԪ����Ԫ�����ڱ��е�λ��Ϊ_______________��
��2��д������ʽ����������ָ��������ѧ�������ͣ�W�ĵ���_____________�� ��;
X��W��ԭ�Ӹ�����2:1�γɵĻ�����_____________�� ��.
��3��W��M��X��Y��Z��Qԭ�Ӱ뾶��С�����˳��Ϊ_____________________(��Ԫ�ط��ű�ʾ)��
��4��W��M��Q�γɵ��⻯����ȶ���__________________________(���⻯�����ʽ��ʾ)
ZԪ���⻯��ķ��ӿռ乹��___________________��
��5��X��Y��Q������������Ӧ��ˮ����֮���������ɷ�Ӧ��д����Ӧ�����ӷ���ʽ��
________________________,________________________,______________________;
��6��д��һ����ѧ��Ӧ����ʽ��֤�����н��ۣ�
�ǽ�����M��Wǿ��__________________________________________________________��
�ǽ�����Q��Zǿ��______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ����һ�и�һ��ѧ�����м�⻯ѧ�Ծ����������� ���ͣ������
��16�֣�W��M��X��Y��Z��Q��ԭ��������������Ķ�����Ԫ�أ�X��Y�ǽ���Ԫ�أ�X����ɫ�ʻ�ɫ������Ԫ�غ˵����֮��Ϊ71��W��Q������������ͬ��Q�ĺ˵������W��2����Z�ĵ��ʺ��������Ϊԭ�Ӿ��塣��ҵ��һ��ͨ�����������ķ������Y�ĵ��ʡ���ش��������⣺
��1��Y�����ӽṹʾ��ͼ_____________;MԪ����Ԫ�����ڱ��е�λ��Ϊ_______________��
��2��д������ʽ����������ָ��������ѧ�������ͣ�W�ĵ���_____________�� ��;
X��W��ԭ�Ӹ�����2:1�γɵĻ����� _____________�� ��.
��3��W��M��X��Y��Z��Qԭ�Ӱ뾶��С�����˳��Ϊ_____________________(��Ԫ�ط��ű�ʾ)��
��4��W��M��Q�γɵ��⻯����ȶ���__________________________(���⻯�����ʽ��ʾ)
ZԪ���⻯��ķ��ӿռ乹��___________________��
��5��X��Y��Q������������Ӧ��ˮ����֮���������ɷ�Ӧ��д����Ӧ�����ӷ���ʽ��
________________________,________________________,______________________;
��6��д��һ����ѧ��Ӧ����ʽ��֤�����н��ۣ�
�ǽ�����M��Wǿ��__________________________________________________________��
�ǽ�����Q��Zǿ��______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ��������������ѧ��һ3���¿���ѧ�Ծ����������� ���ͣ������
��15�֣�W��M��X��Y��Z��Q��ԭ��������������Ķ�����Ԫ�أ�X��Y�ǽ���Ԫ�أ�X����ɫ�ʻ�ɫ������Ԫ�غ˵����֮��Ϊ71��W��Q������������ͬ��Q�ĺ˵������W��2����Z�ĵ��ʺ��������Ϊԭ�Ӿ��塣��ҵ��һ��ͨ�����������ķ������Y�ĵ��ʡ���ش��������⣺
(1)Y�����ӽṹʾ��ͼ____________��MԪ����Ԫ�����ڱ��е�λ��Ϊ______________��
(2)д������ʽ����������ָ��������ѧ�������ͣ�W�ĵ���______________�� ����
X��W��ԭ�Ӹ�����2:1�γɵĻ�����_____________�� ����
(3)W��M��X��Y��Z��Qԭ�Ӱ뾶��С�����˳��Ϊ__________________(��Ԫ�ط��ű�ʾ)��
(4)W��M��Q�γɵ��⻯����ȶ���_______________________(���⻯�����ʽ��ʾ)��ZԪ���⻯��ķ��ӿռ乹��___________________��
(5)X��Y��Q������������Ӧ��ˮ����֮���������ɷ�Ӧ��д����Ӧ�����ӷ���ʽ��
________________________��________________________��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ��һ��ѧ�����м�⻯ѧ�Ծ��������棩 ���ͣ������
��16�֣�W��M��X��Y��Z��Q��ԭ��������������Ķ�����Ԫ�أ�X��Y�ǽ���Ԫ�أ�X����ɫ�ʻ�ɫ������Ԫ�غ˵����֮��Ϊ71��W��Q������������ͬ��Q�ĺ˵������W��2����Z�ĵ��ʺ��������Ϊԭ�Ӿ��塣��ҵ��һ��ͨ�����������ķ������Y�ĵ��ʡ���ش��������⣺
��1��Y�����ӽṹʾ��ͼ_____________;MԪ����Ԫ�����ڱ��е�λ��Ϊ_______________��
��2��д������ʽ����������ָ��������ѧ�������ͣ�W�ĵ���_____________�� ��;
X��W��ԭ�Ӹ�����2:1�γɵĻ����� _____________�� ��.
��3��W��M��X��Y��Z��Qԭ�Ӱ뾶��С�����˳��Ϊ_____________________(��Ԫ�ط��ű�ʾ)��
��4��W��M��Q�γɵ��⻯����ȶ���__________________________(���⻯�����ʽ��ʾ)
ZԪ���⻯��ķ��ӿռ乹��___________________��
��5��X��Y��Q������������Ӧ��ˮ����֮���������ɷ�Ӧ��д����Ӧ�����ӷ���ʽ��
________________________,________________________,______________________;
��6��д��һ����ѧ��Ӧ����ʽ��֤�����н��ۣ�
�ǽ�����M��Wǿ��__________________________________________________________��
�ǽ�����Q��Zǿ��______________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com