
����ͼ��ʾװ�ý����к��Ȳⶨʵ�飬��ش��������⣺
�š���С�ձ�֮����������ĭ���ϵ������� �� ����ʵ��װ���Ͽ���ͼ��ȱ�ٵ�һ�ֲ��������� �� ��
�ơ�ʹ�ò�ȫ�������װ�ý���ʵ�飬ȡ50mL0.25mol��LH2SO4��Һ�� 50mL0.55 mol��L NaOH��Һ��С�ձ��н����кͷ�Ӧ������ʵ���¶�ƽ������3.4�档��֪�кͺ����ɵ���Һ�ı�����cΪ 4.18J�� (g����)����Һ���ܶȾ�Ϊ1g��cm3��ͨ������ɵ��к���
��H= �� �� H2SO4��NaOH��Ӧ���Ȼ�ѧ����ʽ �� ��
�ǡ�ʵ��������60mL0��25mol��L��1H2SO4��Һ��50mL0��55mol��L��1NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� �� (���ȡ���������ȡ�)�������к��� �� (���ȡ���������ȡ�)������50mL0��50mol��L��1�������H2SO4��Һ��������ʵ�飬��÷�Ӧǰ���¶ȵı仯ֵ�� �� ��(�ƫ����ƫС����������Ӱ�족)
�ȡ�����NaOH��Һ��Ϊ��ͬ�������ͬŨ�ȵİ�ˮ������к���Ϊ��H 1 ����H1�릤H�Ĺ�ϵΪ����H 1 �� ��H�����������������=������������ �� ��
 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У�
����ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У�| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У�
����ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У�| ˮ�� |
| �� |
| ˮ�� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ�����IJ���ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��9�֣�����ͼ��ʾװ�ý����к��Ȳⶨʵ�飬��ش��������⣺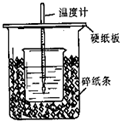
��1����С�ձ�֮����������ĭ���ϵ������� ����ʵ��װ���Ͽ���ͼ��ȱ�ٵ�һ�ֲ��������� ��
��2��ʹ�ò�ȫ�������װ�ý���ʵ�飬ȡ50mL 0��25mol��L H2SO4��Һ��50mL0��55 mol��L NaOH��Һ��С�ձ��н����кͷ�Ӧ������ʵ���¶�ƽ������3��4�档��֪�кͺ����ɵ���Һ�� ������Ϊ4��18J����g���棩����Һ���ܶȾ�Ϊ1g��cm3��ͨ������ɵ��к��ȡ�H= ��
H2SO4��NaOH��Ӧ���Ȼ�ѧ����ʽΪ ��
��3��ʵ��������60mL0��25mol��L��1H2SO4��Һ��50mL0��55mol��L��1NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� �����ȡ���������ȡ����������к��� �����ȡ���������ȡ���������50mL0��50mol��L��1�������H2SO4��Һ��������ʵ�飬��÷�Ӧǰ���¶ȵı仯ֵ�� ���ƫ����ƫС����������Ӱ�족����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com