
��ͼ��ʵ�����Ʊ�1��2���������鲢����һϵ�����ʵ���װ�ã����ȼ��г��豸���ԣ���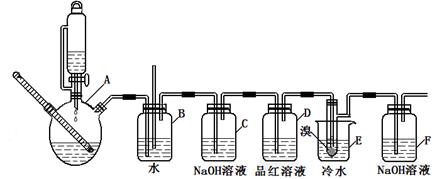
�й������б����£�
| | �Ҵ� | 1,2-�������� | ���� |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶȣ�g/cm3 | 0.79 | 2.2 | 0.71 |
| �е㣯�� | 78.5 | 132 | 34.6 |
| �۵㣯�� | һl30 | 9 | -1l6 |
��1��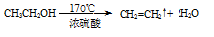 ��2�֣�
��2�֣�
��2��ʹ��ͨ��Һ©�������Һ�����£�2�֣�
��3����֤���������Ƿ���;��������Һ��������2�֣���
��4������Һ��ӷ�;1,2����������������ɹ��������������2�֣�
��5��E��Һ���ɺ���ɫ��Ϊ��ɫ;����Ӧ������Ӧ̫���ң�2�֣���
��6����;��ϩ��Һ������������Ȼ�̼��2�֣�
���������������1���ڼ��Ⱥʹ������������Ҵ�������ȥ��Ӧ������ϩ��Ȼ����ϩ����ˮ�����ӳɷ�Ӧ����1��2���������飬��Ӧ�Ļ�ѧ����ʽ��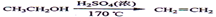 ��
��
��2�����巢��װ��ʹ����ͨ��Һ©������Ҫ����Һ�����µĴ���ѹǿ��ͬ��ʹ��ͨ��Һ©�������Һ�����¡�
��3��װ��D��Ʒ����Һ����������֤���������Ƿ���;��ͬʱBװ���ǰ�ȫƿ����E�з�����������������Һ��϶���������
��4����Ӧ������Ӧ����ˮ��ȴװ��E������ҪĿ���Ǽ���Һ��ӷ�;�����ֲ��ܹ�����ȴ(���ñ�ˮ)����ԭ����1,2����������������ɹ��������������
��5���жϸ��Ʊ���Ӧ�Ѿ������ķ�����E��Һ���ɺ���ɫ��Ϊ��ɫ�����ѧ�����ַ�Ӧ����ʱ����ˮ�Ҵ��������������ֵ����ԭ���Ǹ���Ӧ�������������ѻ�Ӧ̫����ʹ�����Ҵ��ӷ���
��6����ϩ��Һ������������Ȼ�̼���ɽ��г�����գ��ʿ��С�
���㣺����1��2�����������Ʊ����й�ʵ��̽����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������;�㷺����������ʳƷ���ʼ���ʳƷ���Ӽ����л��ϳ��м���ȡ���һ�ֺϳ�ԭ�����£�
��ʵ�鲽�衿
����A�����μ����ʯ��һ�������ı���ȩ��������������̼��ء�
�ڿ����¶�1500C~1700C��ʹ���ַ�Ӧ��
������ȴ�������ƿ�ڼ��뱥��̼������Һ������pH��9~10��
����װ��B��ʾ����ˮ��������ȥδ��Ӧ�ı���ȩ��
�ݼ������̿������������ɫ��
�ޡ���
��1��װ��A���������� �ˣ��a����b����ͨ������ˮ��
��2��������м��뱥��̼������Һ������ᡢ����ת��Ϊ������ƺʹ����Ƶ�ԭ�� ��
��3��װ��B�ڽ���ˮ��������֮ǰ������еIJ���Ϊ �������ܵ�����Ϊ ��
��4�����۲쵽�������� ��˵��ˮ�������������
��5�������ͨ�����²������롢�ᴿ�ýϴ���������ᣨ������ˮ��������ȷ�IJ���˳���� ������ĸ����
a���ؽᾧ b����ȴ�����ˣ�ˮϴ����
c������Ũ�������pH=3 d�����ã����ȹ��˵����������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��λ�ڢ�A�壬��±���������ʽṹ��ѧ���о��ȵ㣬Ҳ����Ҫ�Ļ���ԭ�ϡ����Ȼ���(BCl3)��������ȡ������(B2H6)��Ҳ�����л��ϳɵĴ�����
���������ϡ���BCl3�ķе�Ϊ12.5 �棬�۵�Ϊ��107.3 �棻��2B��6HCl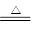 2BCl3����3H2���������������������ƣ�Ҳ��������������Һ��Ӧ��
2BCl3����3H2���������������������ƣ�Ҳ��������������Һ��Ӧ��
�����ʵ�顿��ijͬѧ�����ͼ��ʾװ���Ʊ����Ȼ���
��ش��������⣺
(1)�����£�������ع����ĩ��Ũ���ᷢ���ķ�Ӧ�����Aװ���еķ�Ӧ��������Ӧ�IJ������̵ļ�̬��ͬ��д��������ع����ĩ��Ũ���ᷴӦ�����ӷ���ʽ��___________________________________��
(2)Eװ�õ�������_______________________________________________��
�����ȥBװ�ã����ܵĺ����______________________________________
(3)д��Dװ���з�����Ӧ�Ļ�ѧ����ʽ��_____________________________
ʵ���п�����һ��ʢװ________(���Լ�����)�ĸ���ܴ���F��Gװ�ã�ʹʵ�����㡣
(4)���Ȼ�����ˮ���ҷ�Ӧ��������(H3BO3)�Ͱ�����д���÷�Ӧ�Ļ�ѧ����ʽ��__________________________________________________________��
ʵ���ұ������Ȼ����ע��������_________________________________��
(5)Ϊ��˳�����ʵ�飬��ȷ�IJ�����________(�����)��������ԭ��________��
���ȵ�ȼA���ƾ��ƣ����ȼD���ƾ���
���ȵ�ȼD���ƾ��ƣ����ȼA���ƾ���
��ͬʱ��ȼA��D���ƾ���
(6)�������һ������ʵ�飬��֤�ƵõIJ�Ʒ���Ƿ�����ۣ�________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����þ[Mg(ClO3)2]����������������ݼ��ȣ�ʵ�����Ʊ�����Mg(ClO3)2��6H2O���������£�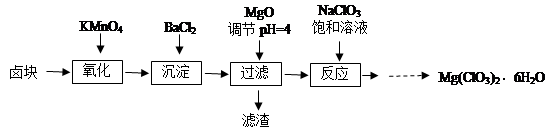
��֪����±����Ҫ�ɷ�ΪMgCl2��6H2O������MgSO4��FeCl2�����ʡ�
�����ֻ�������ܽ��(S )���¶�(T )�仯������ͼ��ʾ��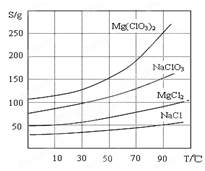
��1����������Ҫ����Ҫ���������� ��
��2������BaCl2��Ŀ���� ����MgO�����������������Ҫ�ɷ�Ϊ ��
��3������NaClO3������Һ������Ӧ�Ļ�ѧ����ʽΪ
��4����Ʒ��Mg(ClO3)2��6H2O�����IJⶨ��
����1��ȷ����3.50 g��Ʒ���100 mL��Һ��
����2��ȡ10.00 mL����ƿ�У�����10.00 mLϡ�����20 .00mL 1.000 mol��L��1��FeSO4��Һ���ȡ�
����3����ȴ�����£���0.100 mol��L��1 K2Cr2O7 ��Һ�ζ�ʣ���Fe2�����յ㣬�˹����з�Ӧ�����ӷ���ʽΪ��Cr2O72����6Fe2����14H����2Cr3����6Fe3����7H2O��
����4��������2��3�ظ����Σ�ƽ������K2Cr2O7 ��Һ15.00 mL��
��д������2�з�����Ӧ�����ӷ���ʽ�� ��
�ڲ�Ʒ��Mg(ClO3)2��6H2O����������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����к�̼�����Ԫ�ء���ѧ��ȤС���ij������Ʒ������̽��������Ҫ��ش��������⡣
��.������̼����Ԫ�صĶ��Լ���
����ͼװ�ý���ʵ��(�г���������ȥ,��ӿ���ĸ����)����ʵ��̼����Ԫ�صļ��顣
(1)����X��������������;װ�âۢ����Լ���ͬ,װ�â���ʢ�ŵ��Լ����� ������
(2)д�����з�Ӧ�Ļ�ѧ����ʽ����������������������������������������
(3)�������װ�âۢܢ�,�ܷ�ȷ��������Ʒ��̼Ԫ�صĴ���?��������,������������������������������������������������
��.������̼�������������IJⶨ
(4)��ͬѧ��Ϊ,����װ�ÿ��Դ��Բⶨ��Ʒ��̼�ĺ�������ȡ��Ʒw1 g����ʵ��,��ַ�Ӧ��,���װ�â������ɵij���Ϊw2 g,����Ʒ��̼����������Ϊ������(�ú�w1��w2��ʽ�ӱ�ʾ)��
(5)��ͬѧ��Ϊ,��һ������Ʒ��ַ�Ӧ��,��װ�â��м�������Ȼ�����Һ,���ݳ����������Լ�����Ʒ�������������,�˷�����õĽ��������(�ƫ��ƫС��);��Ҫ�����Ԫ�غ����IJⶨ����,�ڲ��ı�ʵ��ԭ����ǰ����,���Բ�ȡ��һ�ִ�ʩ������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������ˮ�����������������ؾ���K3[Fe(C2O4)3]��3H2O��������Ӱ����ɫӡˢ������мΪԭ�ϵ��Ʊ��������£�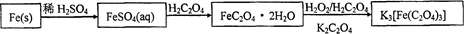
��ش��������⣺
��1����м�г�����Ԫ�أ���ϡ����ʱ������ж���H2S���壬��������������Һ���գ���������װ����ȷ���� ��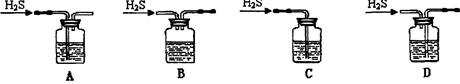
��2���Ƶõ�FeSO4��Һ�������������H2SO4�ữ��Ŀ���� ����Ҫ����Һ�еõ��̷�FeSO4��7H2O��������е�ʵ������� ����˳����д����
a������ϴ�� b������Ũ�� c����ȴ�ᾧ d������ e������
��3���þ�������110�����ȫʧȥ�ᾧˮ�����������¶ȿɷ����ֽⷴӦ��
�ٷֽ�õ����������������װ�ý���ʵ��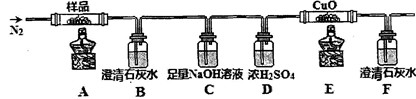
װ�ü�������Ժ���ͨһ��ʱ��N2����Ŀ��Ϊ ������ʵ��ʱ��Ϩ��ƾ�����ͨ��N2�����£���Ŀ��Ϊ ��ʵ������й۲쵽B��F�г���ʯ��ˮ������ǣ�E���к�ɫ�������ɣ������������ ��
�ڷֽ�õ��Ĺ�����ﺬ��K2CO3��FeO��Fe����ˮ�ܽ⡢���ˡ�ϴ�ӡ�����õ�������Ʒ���������������ʵ�鷽���Ը���Ʒ�������ʺ����ⶨ��
���ס�a g��Ʒ ��Һ
��Һ
 �ù���b g
�ù���b g
���ҡ�a g��Ʒ
 ��������������Va mL
��������������Va mL
������a g��Ʒ 250 mL��Һ
250 mL��Һ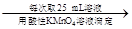 ����ƽ������0.1 mol��L��1����KMnO4��ҺVb mL����Ϊ���Ϸ����� ��ȷ����Ʒ����ɣ������� ��
����ƽ������0.1 mol��L��1����KMnO4��ҺVb mL����Ϊ���Ϸ����� ��ȷ����Ʒ����ɣ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��̼���ƣ�2Na2CO3��3H2O2����һ�ּ�ϴ�ӡ�Ư�ס�ɱ����һ�����ϵƯ�����þ������Na2CO3��H2O2��˫�����ʡ�����ͼ-2װ���Ʊ���̼���ƣ�����ˮԡ�г�ַ�Ӧ��ͼ-1���̿ɻ�ù�̼���Ʋ�Ʒ��

��1����ѹ��Һ©����֧�ܵ������� ��
��2���Ʊ���̼���ƵĹؼ��� ��
��3��������ƹ�̼���Ƶ�ˮ�к��������ӣ�����������ϴ�Ӽ���ȥ��������������ȫʧȥɱ�����á��Է������е�ԭ��д������һ�ּ��ɣ��÷���ʽ��ʾ����________________________________��
��4��ij��ѧѧϰС��Ϊ�˶���̽�������Ӷ���������Ư���IJ���Ӱ�죬ȡ��Ư��100mL������25g FeCl3���壬����������ɫ��ζ���壬������ƿ�ռ����塣��ѡ�������Լ���ʵ����Ʒ�������ɷֵ�̽�����̣�0��1mol/LNaOH��Һ��8��0mol/LNaOH��Һ������ʯ��ˮ��0��01mol/LKMnO4��Һ��BaCl2ϡ��Һ��Ʒ����Һ������ˮ��ľ�����ƾ��ơ����ϴ��ƿ��
��������裺�Ը�����ɷ�����������衣
����1��������O2�� ����2��������______________�� ����3��������CO2��
����Ʒ��������ʵ�鷽��֤����ļ��裬���±������ʵ�鲽�衢Ԥ����������ۣ�
| ʵ�鲽�� | Ԥ����������� |
| ����������ͨ��ʢ��_______��________��ϴ��ƿ�У�________________________�� | ��________________________ ��________________________ ��________________________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС����̽���ں�Mg2+��Al3+ �Ļ����Һ�еμ�NaOH��Һʱ������������������Ĺ��̡�
��ʵ�顿��0.1 mol?L��1 MgSO4��0.05 mol?L��1Al2(SO4)3�Ļ����Һ�еμ�0.5 mol?L-1NaOH��Һ�����������Ӵ���������Ӧ���������������Һ��pH��NaOH��Һ�ļ���仯�����ͼ��ʾ��
��1��Ϊ��ȷ���Ƽ���NaOH��Һ��������ɽ�NaOH��Һ���� �����������ƣ��еμӡ�
��2��ͼ��������pH���ӻ����ĽΣ���һ�Σ�a��ǰ����Ӧ��ʵ�������� ��
��3���Եڶ��Σ�b��c֮�䣩��ҺpH�仯�����ı��ʣ�С��ͬѧ���������Ʋ⣬�벹���Ʋ�2��3��
�Ʋ�1������Mg(OH)2����������OH����
�Ʋ�2�� ��
�Ʋ�3�� ��
���Ʋ�1����ʵ���������a��֮ǰ��Ӧ�����ӷ���ʽΪ ����ݴ�����Mg(OH)2��Al(OH)3����������ˮ��Һ���ܽ��ԵIJ��� ��
��4�������e�����Һ�нϴ������ڵĺ�����Ԫ�ص����Ӳ����ʵ����飨�ɲ���������
| �ϴ������ڵĺ�����Ԫ�ص����� | ���鷽�� |
| | |
| | |
| | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������һ���ս̰桶��ѧ1�����ޣ�����ҵ�ϴӺ���Ʒ���������л�ȡ�������ͼ���£�
��1�������������̵ĵ�һ���ǡ����ݡ�������ʵ��Ŀ���� ��
��2������������һ�����пɹ�ѡ�õ��Լ���Cl2��Br2��Ũ�����H2O2���μ�ϡ���ᣩ��������Ⱦ�Ƕȿ��ǣ�����ѡ��ĺ����Լ� ��
��3�����������������У����ᴿ��õ��ʵ⡱һ����Ϊ��������������ΪӦѡ����ͼʵ��װ�÷ֱ��ǣ� ���� �� �����������Ⱥ�˳����д����


 �����϶����ҹ�ʵ�ʹ�ҵ���ú�����ȡ�⣬���õ������ӽ����������������£�
�����϶����ҹ�ʵ�ʹ�ҵ���ú�����ȡ�⣬���õ������ӽ����������������£�
��4��ʵ�ʹ�ҵ�����У��ữ�������ķ������ȼ��������ữ��ʹpH���͵�2��Ȼ������������һ�����������ʹ��������������ҵͨ��������������ԭ���ǣ������ӷ���ʽ��ʾ��
��
��5���������������������ӽ�����֬���ü�����֬������������������һ�ԭ��������������֬�����ĵ�Ԫ��״̬�� ����д������̬������̬������������Ӧ���� ����д���б�ţ�A����������B����ԭ��������ʵ�ֵ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com