
| A�� | ��a=b��pH��NaX����pH��NaY��������ͬŨ��ʱ������HX��HY | |
| B�� | ��a=b���������Һ��c��X-��=c��Y-��+c��HY����c��HY����0��������ͬŨ��ʱ������HX��HY | |
| C�� | ��a��b���������Һ��c��HX��=c��HY��������Ƴ���Һ��c��X-����c��Y-��������ͬŨ��ʱ������HX��HY | |
| D�� | ������Һ�������ϣ������Һ��c��X-��+c��Y-��+c��HX��+c��HY��=0.1 mol/L������Ƴ�a+b=0.2 mol/L |
���� A����ͬŨ�ȵ�������Һ����Һ�ļ���Խǿ������������Խ����
B����a=b�������c��X-��=c��Y-��+c��HY����˵����ͬŨ��ʱHX�ĵ���̶ȴ���HY��
C����a��b���������Һ��c��HX��=c��HY�������c��X-����c��Y-����˵��HX�ĵ���̶ȴ���HY��
D�����������Һ�������ϣ���Һ�������һ������Ũ�Ƚ�Ϊԭ����һ�룮
��� �⣺A����ͬŨ�ȵ�������Һ����Һ�ļ���Խǿ������������Խ����������a=b��pH��NaX����pH��NaY����˵��HX������С��HY����������ͬŨ��ʱ������HX��HY����A����
B����a=b�������c��X-��=c��Y-��+c��HY����˵����ͬŨ��ʱHX�ĵ���̶ȴ���HY������ͬŨ��ʱ����ĵ���̶�Խ��������Խǿ����������HX��HY����B��ȷ��
C����a��b���������Һ��c��HX��=c��HY�������c��X-����c��Y-����˵��X-ˮ��̶�С��Y-��HX�ĵ���̶ȴ���HY����ͬŨ��ʱ������HX��HY����C��ȷ��
D�����������Һ�������ϣ���Һ�������һ������Ũ�Ƚ�Ϊԭ����һ�룬������c��X-��+c��Y-��+c��HX��+c��HY��=0.1mol/L������Ƴ�a+b=0.2mol/L����D��ȷ��
��ѡA��
���� ���⿼��������ˮ�⣬������ͬŨ��������ҺpH��Сȷ�����ǿ����֪�����ǿ������Ũ�ȵĹ�ϵ�ǽⱾ��ؼ���ע��D���������һ��ʱ��Ũ�ȱ仯������Ȼ��ѭ�����غ㣬��Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | Fe3+ | B�� | HS- | C�� | 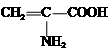 | D�� | C6H5O- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ���к��еĻ�ѧ�����������Ӽ������ۼ�
���к��еĻ�ѧ�����������Ӽ������ۼ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������Ҵ��������������ɱ���������ˮ���Ʊ�������Ҵ� | |
| B�� | �ɼױ������ƶ������ױ����ɼױ������Ʊ����� | |
| C�� | ���ȴ���������ȥ�ƻ���ϩ���ɱ�ϩ������1��2������� | |
| D�� | �������ˮ���Ʊ������ɱ�ϩ��ˮ��Ӧ�Ʊ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
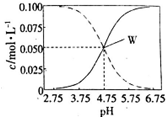 �����������ڴ��ᡢ�����ƻ����Һ�У���c��CH3COOH��+c��CH3COO-��=0��lmol��L-1ʱ��c��CH3COOH����c��CH3COO-����pH�Ĺ�ϵ��ͼ��ʾ�������й�������ȷ���ǣ�������
�����������ڴ��ᡢ�����ƻ����Һ�У���c��CH3COOH��+c��CH3COO-��=0��lmol��L-1ʱ��c��CH3COOH����c��CH3COO-����pH�Ĺ�ϵ��ͼ��ʾ�������й�������ȷ���ǣ�������| A�� | pH=5.5����Һ�У�c��CH3COOH����c��CH3COO-����c��H+����c��OH-�� | |
| B�� | ��W������ʾ��1.0L��Һ��ͨ��0.05molHCl���壨��Һ����仯�ɺ��ԣ���c��H+��=c��CH3COOH��+c��OH-�� | |
| C�� | W������ʾ����Һ�У�c��Na+��+c��H+��=c��CH3COOH��+c��OH��- | |
| D�� | pH=3.5����Һ�У�c��Na+��+c��H+��+c��OH-��+c��CH3COOH��=0.1mol��L-l |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
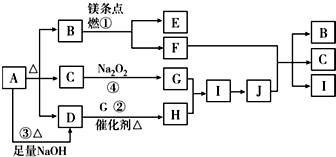
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
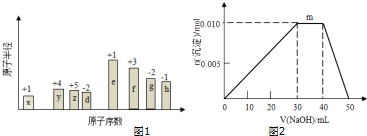
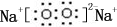 ��0.10mol�û�����������ˮ��Ӧʱת�Ƶĵ�����Ϊ6.02��1022��
��0.10mol�û�����������ˮ��Ӧʱת�Ƶĵ�����Ϊ6.02��1022��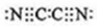 ����yz��2��Ϊ��±�أ�������±�����ƣ���������������Һ��Ӧ�Ļ�ѧ����ʽ��2NaOH+��CN��2=NaCN+NaCNO+H2O��
����yz��2��Ϊ��±�أ�������±�����ƣ���������������Һ��Ӧ�Ļ�ѧ����ʽ��2NaOH+��CN��2=NaCN+NaCNO+H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ͷ۾���NaHCO3��ʹ������ʹ������ʳƷ���ɿɿ� | |
| B�� | ȼú������������CaO�������ڽ��������γɵĸ��� | |
| C�� | ���ýϾõĺ��������ڳ������𣬿����������ǵ�ˮ���й� | |
| D�� | ��Һ���������㽶��������ϩ���ﵽ���ʵ�Ŀ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com