
��13�֣�����1�������仯�����ڹ�ũҵ���������й㷺��Ӧ�á�������أ�K2FeO4�����м�ǿ�������ԣ���һ��������ˮ��������
��1����֪��4FeO42����10H2O![]() 4Fe(OH)3��8OH����3O2��
4Fe(OH)3��8OH����3O2��
K2FeO4�ڴ���ˮ�Ĺ���������������� ��
��2��������K2FeO4�ܽ���pH��4.74����Һ�У����Ƴ�c(FeO42��) ��1.0 mmol��L��1���������������ֱ�����20�桢30�桢40���60��ĺ���ˮԡ�У��ⶨc(FeO42��)�ı仯�������ͼ��ʵ���Ŀ���� ��������Ӧ�ġ�H 0�����������������������
��3��FeO42����ˮ��Һ�еĴ�����̬��ͼ����ʾ������˵����ȷ���� ������ĸ����
A��������Һ�������α仯����Ԫ�ض���4�ִ�����̬
B����pH��10��������Һ�м�������pH��2��HFeO4���ķֲ�����������
C����pH��6��������Һ�м�KOH��Һ��������Ӧ�����ӷ���ʽΪ��
HFeO4����OH����FeO42����H2O
����2��������KxFe(C2O4)y��zH2O(FeΪ+3��)��һ�ֹ����в��ϣ�ʵ���ҿ��������·����Ʊ����ֲ��ϲ��ⶨ����ɡ�
I���Ʊ���
��4���ᾧʱӦ��������Һ�ںڰ����ȴ����������������������ԭ���� ��
��5��������������� ��
����ɲⶨ��
��ȡ0.491gʵ�����þ���(�����Ǵ�����)������ƿ�У�����������ˮ��ϡH2SO4����C2O42-��ȫת��ΪH2C2O4����0��10mol��L-1KMnO4��Һ���еζ�������KMnO4��Һ12��00mLʱǡ�÷�Ӧ���ټ��������Ļ�ԭ������Fe3+��ȫת��ΪFe2+����KMnO4��Һ�����ζ�����Fe2+��ȫ����ʱ����ȥKMnO4��Һ2��00mL����ط�Ӧ���£�
2KMnO4+5H2C2O4+3H2SO4=2MnSO4+K2SO4+10CO2��+8H2O
MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O
��6������250mL 0��10mol��L-1KMnO4��Һ�������ζ�ʵ��������IJ����������ձ�������������ͷ�ιܡ���Ͳ����ƿ��� �� �������ζ��е����յ�ʱ��Һ��ɫΪ ɫ����30���ڲ���ɫ��
��7��ͨ�����㣬��˹������ϵĻ�ѧʽ ��
����13�֣�
��1��ɱ����������������������������𰸣�����1�֣���2�֣�
��2��̽���¶ȶ�FeO42��Ũ�ȵ�Ӱ�죨�����������𰸣���1�֣� ����1�֣�
��3��C��1�֣�
��4���ڰ����Է�ֹ����ֽ⣨1�֣� ��5�����ˡ�ϴ�ӣ���1�֣���2�֣�
��6��250mL����ƿ����ʽ�ζ��ܣ�2�֣� �Ϻ죨1�֣� (7) K3Fe(C2O4)3��3H2O��2�֣�
����:��1��K2FeO4�е���Ԫ�ػ�����ߣ��к�ǿ�������Ծ���ɱ�������ã�����ˮ������ɽ��壬�������������������ˮ��
��2����3������ͨ��ͼ���ռ���Ϣ��������⼰֪ʶ�Ļ��۽��и����ܽᡣ�������͡�
��4����5����6������һЩ������ʵ�����
��7��MnO4- + 5Fe2+ + 8H+ = Mn2++5Fe3++4H2O
0.0002mol 0.001mol
2KMnO4 + 5H2C2O4+3H2SO4=2MnSO4+K2SO4+10CO2��+8H2O
0.0012mol 0.003mol
�ʣ���֪��ѧʽKxFe(C2O4)y��zH2O��y =3���ɻ��ϼ۽�һ����֪x=3
��0.491g��ȥKxFe(C2O4)y���������������H2O ������Ϊ0.054g����0.003mol



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��13�֣�����1�������仯�����ڹ�ũҵ���������й㷺��Ӧ�á�������أ�K2FeO4�����м�ǿ�������ԣ���һ��������ˮ��������
��1����֪��4FeO42����10H2O![]() 4Fe(OH)3��8OH����3O2��
4Fe(OH)3��8OH����3O2��
K2FeO4�ڴ���ˮ�Ĺ���������������� ��
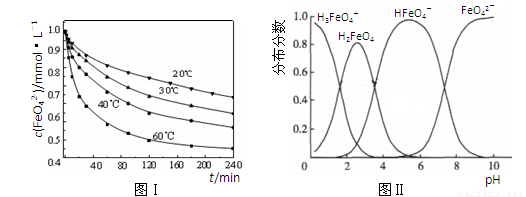
��2��������K2FeO4�ܽ���pH��4.74����Һ�У����Ƴ�c(FeO42��) ��1.0 mmol��L��1���������������ֱ�����20�桢30�桢40���60��ĺ���ˮԡ�У��ⶨc(FeO42��)�ı仯�������ͼ��ʵ���Ŀ���� ��������Ӧ�ġ�H 0�����������������������
��3��FeO42����ˮ��Һ�еĴ�����̬��ͼ����ʾ������˵����ȷ���� ������ĸ����
A��������Һ�������α仯����Ԫ�ض���4�ִ�����̬
B����pH��10��������Һ�м�������pH��2��HFeO4���ķֲ�����������
C����pH��6��������Һ�м�KOH��Һ��������Ӧ�����ӷ���ʽΪ��
HFeO4����OH����FeO42����H2O
����2��������KxFe(C2O4)y��zH2O(FeΪ+3��)��һ�ֹ����в��ϣ�ʵ���ҿ��������·����Ʊ����ֲ��ϲ��ⶨ����ɡ�
I���Ʊ���
��4���ᾧʱӦ��������Һ�ںڰ����ȴ����������������������ԭ���� ��
��5��������������� ��
����ɲⶨ��
��ȡ0.491gʵ�����þ���(�����Ǵ�����)������ƿ�У�����������ˮ��ϡH2SO4����C2O42-��ȫת��ΪH2C2O4����0��10mol��L-1KMnO4��Һ���еζ�������KMnO4��Һ12��00mLʱǡ�÷�Ӧ���ټ��������Ļ�ԭ������Fe3+��ȫת��ΪFe2+����KMnO4��Һ�����ζ�����Fe2+��ȫ����ʱ����ȥKMnO4��Һ2��00mL����ط�Ӧ���£�
2KMnO4+5H2C2O4+3H2SO4=2MnSO4+K2SO4+10CO2��+8H2O
MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O
��6������250mL 0��10mol��L-1KMnO4��Һ�������ζ�ʵ��������IJ����������ձ�������������ͷ�ιܡ���Ͳ����ƿ��� �� �������ζ��е����յ�ʱ��Һ��ɫΪ ɫ����30���ڲ���ɫ��
��7��ͨ�����㣬��˹������ϵĻ�ѧʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ���ݵڶ���ѧ�߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��13�֣�����1�������仯�����ڹ�ũҵ���������й㷺��Ӧ�á�������أ�K2FeO4�����м�ǿ�������ԣ���һ��������ˮ��������
��1����֪��4FeO42����10H2O 4Fe(OH)3��8OH����3O2��
4Fe(OH)3��8OH����3O2��
K2FeO4�ڴ���ˮ�Ĺ���������������� ��
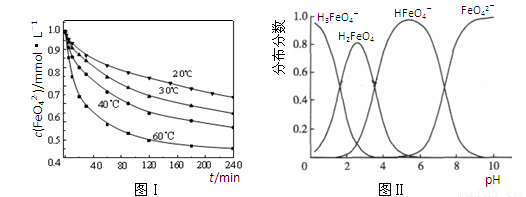
��2��������K2FeO4�ܽ���pH��4.74����Һ�У����Ƴ�c(FeO42��) ��1.0 mmol��L��1���������������ֱ�����20�桢30�桢40���60��ĺ���ˮԡ�У��ⶨc(FeO42��)�ı仯�������ͼ��ʵ���Ŀ���� ��������Ӧ�ġ�H 0�����������������������
��3��FeO42����ˮ��Һ�еĴ�����̬��ͼ����ʾ������˵����ȷ���� ������ĸ����
A��������Һ�������α仯����Ԫ�ض���4�ִ�����̬
B����pH��10��������Һ�м�������pH��2��HFeO4���ķֲ�����������
C����pH��6��������Һ�м�KOH��Һ��������Ӧ�����ӷ���ʽΪ��
HFeO4����OH����FeO42����H2O
����2��������KxFe(C2O4) y��zH2O(FeΪ+3��)��һ�ֹ����в��ϣ�ʵ���ҿ��������·����Ʊ����ֲ��ϲ��ⶨ����ɡ�
I���Ʊ���
��4���ᾧʱӦ��������Һ�ںڰ����ȴ����������������������ԭ���� ��
��5��������������� ��
����ɲⶨ��
��ȡ0.491gʵ�����þ���(�����Ǵ�����)������ƿ�У�����������ˮ��ϡH2SO4����C2O42-��ȫת��ΪH2C2O4����0��10mol��L-1KMnO4��Һ���еζ�������KMnO4��Һ12��00mLʱǡ�÷�Ӧ���ټ��������Ļ�ԭ������Fe3+��ȫת��ΪFe2+����KMnO4��Һ�����ζ�����Fe2+��ȫ����ʱ����ȥKMnO4��Һ2��00mL����ط�Ӧ���£�
2KMnO4+5H2C2O4+3H2SO4=2MnSO4+K2SO4+10CO2��+8H2O
MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O
��6������250mL 0��10mol��L-1KMnO4��Һ�������ζ�ʵ��������IJ����������ձ�������������ͷ�ιܡ���Ͳ����ƿ��� �� �������ζ��е����յ�ʱ��Һ��ɫΪ ɫ����30���ڲ���ɫ��
��7��ͨ�����㣬��˹������ϵĻ�ѧʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��13�֣�����1�������仯�����ڹ�ũҵ���������й㷺��Ӧ�á�������أ�K2FeO4�����м�ǿ�������ԣ���һ��������ˮ��������
��1����֪��4FeO42����10H2O 4Fe(OH)3��8OH����3O2��
4Fe(OH)3��8OH����3O2��
K2FeO4�ڴ���ˮ�Ĺ���������������� ��
��2��������K2FeO4�ܽ���pH��4.74����Һ�У����Ƴ�c(FeO42��) ��1.0 mmol��L��1���������������ֱ�����20�桢30�桢40���60��ĺ���ˮԡ�У��ⶨc(FeO42��)�ı仯�������ͼ��ʵ���Ŀ���� ��������Ӧ�ġ�H 0�����������������������
��3��FeO42����ˮ��Һ�еĴ�����̬��ͼ����ʾ������˵����ȷ���� ������ĸ����
A��������Һ�������α仯����Ԫ�ض���4�ִ�����̬
B����pH��10��������Һ�м�������pH��2��HFeO4���ķֲ�����������
C����pH��6��������Һ�м�KOH��Һ��������Ӧ�����ӷ���ʽΪ��
HFeO4����OH����FeO42����H2O
����2��������KxFe(C2O4) y��zH2O(FeΪ+3��)��һ�ֹ����в��ϣ�ʵ���ҿ��������·����Ʊ����ֲ��ϲ��ⶨ����ɡ�
I���Ʊ���

��4���ᾧʱӦ��������Һ�ںڰ����ȴ����������������������ԭ���� ��
��5��������������� ��
����ɲⶨ��
��ȡ0.491gʵ�����þ���(�����Ǵ�����)������ƿ�У�����������ˮ��ϡH2SO4����C2O42-��ȫת��ΪH2C2O4����0��10mol��L-1KMnO4��Һ���еζ�������KMnO4��Һ12��00mLʱǡ�÷�Ӧ���ټ��������Ļ�ԭ������Fe3+��ȫת��ΪFe2+����KMnO4��Һ�����ζ�����Fe2+��ȫ����ʱ����ȥKMnO4��Һ2��00mL����ط�Ӧ���£�
2KMnO4+5H2C2O4+3H2SO4=2MnSO4+K2SO4+10CO2��+8H2O
MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O
��6������250mL 0��10mol��L-1KMnO4��Һ�������ζ�ʵ��������IJ����������ձ�������������ͷ�ιܡ���Ͳ����ƿ��� �� �������ζ��е����յ�ʱ��Һ��ɫΪ ɫ����30���ڲ���ɫ��
��7��ͨ�����㣬��˹������ϵĻ�ѧʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ɽ��ʡΫ���и�����ѧ�ڿ�ѧ�����Ի�ѧ���� ���ͣ������
��13�֣�����1�������仯�����ڹ�ũҵ���������й㷺��Ӧ�á�������أ�K2FeO4�����м�ǿ�������ԣ���һ��������ˮ��������
��1����֪��4FeO42����10H2O 4Fe(OH)3��8OH����3O2��
4Fe(OH)3��8OH����3O2��
K2FeO4�ڴ���ˮ�Ĺ���������������� ��
��2��������K2FeO4�ܽ���pH��4.74����Һ�У����Ƴ�c(FeO42��) ��1.0 mmol��L��1���������������ֱ�����20�桢30�桢40���60��ĺ���ˮԡ�У��ⶨc(FeO42��)�ı仯�������ͼ��ʵ���Ŀ���� ��������Ӧ�ġ�H 0�����������������������
��3��FeO42����ˮ��Һ�еĴ�����̬��ͼ����ʾ������˵����ȷ���� ������ĸ����
A��������Һ�������α仯����Ԫ�ض���4�ִ�����̬
B����pH��10��������Һ�м�������pH��2��HFeO4���ķֲ�����������
C����pH��6��������Һ�м�KOH��Һ��������Ӧ�����ӷ���ʽΪ��
HFeO4����OH����FeO42����H2O
����2��������KxFe(C2O4) y��zH2O(FeΪ+3��)��һ�ֹ����в��ϣ�ʵ���ҿ��������·����Ʊ����ֲ��ϲ��ⶨ����ɡ�
I���Ʊ���

��4���ᾧʱӦ��������Һ�ںڰ����ȴ����������������������ԭ���� ��
��5��������������� ��
����ɲⶨ��
��ȡ0.491gʵ�����þ���(�����Ǵ�����)������ƿ�У�����������ˮ��ϡH2SO4����C2O42-��ȫת��ΪH2C2O4����0��10mol��L-1KMnO4��Һ���еζ�������KMnO4��Һ12��00mLʱǡ�÷�Ӧ���ټ��������Ļ�ԭ������Fe3+��ȫת��ΪFe2+����KMnO4��Һ�����ζ�����Fe2+��ȫ����ʱ����ȥKMnO4��Һ2��00mL����ط�Ӧ���£�
2KMnO4+5H2C2O4+3H2SO4=2MnSO4+K2SO4+10CO2��+8H2O
MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O
��6������250mL 0��10mol��L-1KMnO4��Һ�������ζ�ʵ��������IJ����������ձ�������������ͷ�ιܡ���Ͳ����ƿ��� �� �������ζ��е����յ�ʱ��Һ��ɫΪ ɫ����30���ڲ���ɫ��
��7��ͨ�����㣬��˹������ϵĻ�ѧʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com