
��ҵ������ĺ��ķ�Ӧ�� ��2SO2��g��+ O2��g��![]() 2SO3��g�� ��H��0���ش��������⣺
2SO3��g�� ��H��0���ش��������⣺
��1���˷�Ӧ��ƽ�ⳣ������ʽΪK= �������¶ȵ����ߣ�
����ƽ�ⳣ�� �����������С�����䡱����
��2����һ������SO2��g����O2��g������1L�ܱ������У���һ�������´ﵽƽ�⣬���SO2Ϊ0.11mol��O2Ϊ0.05mol��SO3Ϊ0.12mol������������£���Ӧ��ƽ�ⳣ��K= ��
SO2��ת��ΪSO3ת����= ��
��3���������������£����д�ʩ�����������SO2��ת���ʵ���
������ĸ����
A��ͨ������ B���Ƴ����� C������ѹǿ
D����СѹǿE���������
��4����ҵ�������β���к�������SO2 �������ð�ˮ���գ��������ᴦ����
�ٷ�Ӧ�Ļ�ѧ����ʽΪ ��
�������������ŵ���
��
 �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д� ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ɫ��ѧ�ĺ��ľ��Ǵ�Դͷ�ϼ��ٺ�������ҵ�����Ի�������Ⱦ������ʵ��Ķ��������ɫ��ѧ������ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| �¶� �ܽ�� �� |
0�� | 10�� | 20�� | 30�� | 40�� | 50�� |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 |
| NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 |
| NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ��ׯʮ����2012�����9���¿���ѧ���� ���ͣ�022
��ҵ������ĺ��ķ�Ӧ�ǣ�2SO2(g)��O2(g)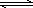 2SO3(g)����H��0���ش��������⣺
2SO3(g)����H��0���ش��������⣺
(1)�˷�Ӧ��ƽ�ⳣ������ʽΪK��________�������¶ȵ����ߣ�����ƽ�ⳣ��________(���������С�����䡱)��
(2)��һ������SO2(g)��O2(g)����1 L�ܱ������У���һ�������´ﵽƽ�⣬���SO2Ϊ0.11 mol��O2Ϊ0.05 mol��SO3Ϊ0.12 mol������������£���Ӧ��ƽ�ⳣ��K��________��
SO2��ת��ΪSO3ת���ʣ�________��
(3)�������������£����д�ʩ�����������SO2��ת���ʵ���________
(����ĸ)��
A��ͨ������
B���Ƴ�����
C������ѹǿ
D����Сѹǿ
E���������
(4)��ҵ�������β���к�������SO2�������ð�ˮ���գ��������ᴦ����
�ٷ�Ӧ�Ļ�ѧ����ʽΪ________��
�������������ŵ���________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0111 ������ ���ͣ������
 2SO3(g) ��H��0���ش��������⣺
2SO3(g) ��H��0���ش��������⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com