
���� ��1��ȼ������1mol��ȼ����ȫȼ�������ȶ�������ʱ�ų�����������25�桢101kPa�£�1g�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����22.68kJ��32g�״�ȼ������CO2��Һ̬ˮʱ����22.68kJ��32=725.76kJ��1mol�״�����Ϊ32�ˣ�������ȫȼ��1mol�״����ɶ�����̼��Һ̬ˮ����725.76KJ������ȼ���ȵĸ���������ɽ��
��2��Al2��SO4��3��ǿ�������Σ�ˮ��Һ��������ˮ����Һ�����ԣ�
��3��������Һ�У������Ӽ���ȫ����������ĵ��룬��������ȫ��������ˮ�ĵ��룻
������Һ�У������Ӻ���������ȫ��������ˮ�ĵ��룻
��4�����������ˮ�⣬��Һ�Լ��ԣ�
��� �⣺��1��1mol�״���ȫȼ�����ɶ�����̼��Һ̬ˮ����725.8KJ��ȼ�����Ȼ�ѧ����ʽΪ��CH3OH��l��+$\frac{3}{2}$O2��g���TCO2��g��+2H2O��l����H=-725.76 kJ•mol-1��
�ʴ�Ϊ��CH3OH��l��+$\frac{3}{2}$O2��g���TCO2��g��+2H2O��l����H=-725.76 kJ•mol-1��
��2��Al2��SO4��3��ǿ�������Σ�ˮ��Һ��������ˮ����Һ�����ԣ����ӷ���ʽΪ��Al3++3H2O?Al��OH��3+3H+��
�ʴ�Ϊ���Al3++3H2O?Al��OH��3+3H+��
��3��25���£�pH=5��������Һ��c��H+��=10-5mol/L��c��OH-��=10-9mol/L����������Һ�У������Ӽ���ȫ����������ĵ��룬��������ȫ��������ˮ�ĵ��룬����ˮ�������c��H+��=c��OH-��=10-9mol/L��
25���£�pH=5���Ȼ����Һ�У�c��H+��=10-5mol/L��c��OH-��=10-9mol/L�������Ȼ����Һ�У������Ӻ���������ȫ��������ˮ�ĵ��룬������笠�����ˮ�⽫������������ϣ�����Һ�е���������ˮ�������ȫ��������ˮ�������c��H+��=c��OH-��=10-5mol/L��
�ʴ�Ϊ��10-9mol/L��10-5mol/L��
��4�����������ˮ�⣬��Һ�Լ��ԣ��������Ӵ����������ӣ������ӹ�ϵΪc��Na+����c��CH3COO-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��CH3COO-����c��OH-����c��H+����
���� ���⿼���Ȼ�ѧ����ʽ��д�������Һ�Ļ�ϡ��漰���롢ˮ��ȣ�ע�ظ߿���������Ŀ��飬���ط�Ӧԭ����ѵ������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ĵ���ʽ | B�� | Mg2+�Ľṹʾ��ͼ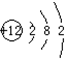 | ||
| C�� | NH3�Ľṹʽ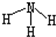 | D�� | ������̼�ĵ���ʽ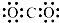 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2O | B�� | HCl | C�� | NH4+ | D�� | OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���백ˮ��������ɫ������֤��Ԫ��Һ�д���Al3+ | |
| B�� | ��AgCl��Һ�еμ�Na2S��Һ����ɫ������ɺ�ɫ��˵��AgCl�ܽ�ƽ�������ƶ� | |
| C�� | ��������Ͷ����ˮ�У�����ΪС��˵������ˮ�ķ�ӦΪ���ȷ�Ӧ���Ƶ��۵�� | |
| D�� | ��Ca��OH��2��Һ�м���������ˮCaO���ָ�ԭ�¶ȣ���Һ��Ca��OH��2��pH���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
�� ��
�� ������A��2��̼ԭ�ӵ�ͬϵ����4��ͬ���칹�壮
������A��2��̼ԭ�ӵ�ͬϵ����4��ͬ���칹�壮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Xֻ���ǵ�������Ԫ�� | B�� | Y�������ǵڶ�����Ԫ�� | ||
| C�� | Y����Ϊ��Ԫ�� | D�� | a-b+m+n����10��20 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com