
|
��֪Ba(AlO2)2������ˮ����ͼ��ʾ������Al2(SO4)3��Һ����μ���Ba(OH)2��Һʱ�����ɳ��������ʵ���y�����Ba(OH)2�����ʵ���x�Ĺ�ϵ�������й�������ȷ����
| |
A�� |
a��bʱ���������ʵ�����Al(OH)3��BaSO4�� |
B�� |
c��dʱ��Һ�����ӵ����ʵ����� |
C�� |
a��dʱ���������ʵ�����BaSO4����С��Al(OH)3 |
D�� |
d��eʱ��Һ�����ӵ����ʵ�����Ba2+���ܵ���OH�� |
|
��0��b��Al3+�� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
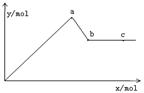
(4�֣���֪Ba(AlO2)2������ˮ����ͼ��ʾ������KA1(SO4)2��Һ����μ���Ba(OH)2��Һʱ�����ɳ��������ʵ���y�����Ba(OH)2�����ʵ���x�Ĺ�ϵ������������⣺
��1��a��ʱ��Ӧ�����ӷ���ʽ��
��2��a��b��������Ba(OH)2�����ʵ���֮����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ�ʡ��ˮ��ѧ����������ģ�⿼�Ի�ѧ�Ծ����������� ���ͣ���ѡ��
��֪Ba(AlO2)2������ˮ������1 mol Al2(SO4)3����Һ�м��뺬��b mol Ba(OH)2 (b��6)����Һ�����ó��������ʵ���������Ϊ
| A��5mol | B��3mol | C��b/2mol | D��5b/3mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���Ϻ��н�ɽ��������ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ���ѡ��
��֪Ba(AlO2)2������ˮ����ͼ��ʾ������A12(SO4)3 0.01mol����Һ����μ���Ba(OH)2��Һʱ�����ɳ��������ʵ���y�����Ba(OH)2�����ʵ���x�Ĺ�ϵ������a��c�ֱ���0b�κ�bd�ε��е㣩�������й�������ȷ����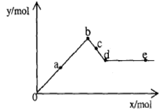
| A��aʱ����������Ϊ3.495 g |
| B��bʱ���������ʵ���Ϊ0.05 mol |
| C��cʱ��Һ��Ba2+���ӵ����ʵ���Ϊ0.005 mol |
| D��eʱ��Һ��AlO2�������ʵ���Ϊ0.01 mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ӱ�ʡ����������ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪Ba(AlO2)2������ˮ������1 mol Al2(SO4)3����Һ�м��뺬��b mol Ba(OH)2 (b��6)����Һ�����ó��������ʵ���������Ϊ
A��5mol B��3mol C��b/2mol D��5b/3mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�������ѧ�ڻ�ѧһ�ָ�ϰ���ӿ��ﵽ�������ϡ�ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ѡ����
��֪Ba(AlO2)2������ˮ����ͼ��ʾ������Al2(SO4)3��Һ����μ���Ba(OH)2��Һʱ�����ɳ��������ʵ���y�����Ba(OH)2�����ʵ���x�Ĺ�ϵ��
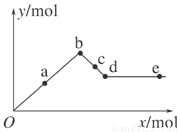
�����й�������ȷ����(����)
A��a��bʱ���������ʵ�����Al(OH)3��BaSO4��
B��c��dʱ��Һ�����ӵ����ʵ�����Ba2����AlO2����
C��a��dʱ���������ʵ�����BaSO4����С��Al(OH)3
D��d��eʱ��Һ�����ӵ����ʵ�����Ba2�����ܵ���OH��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com