
��ijһ�ݻ�Ϊ2 L���ܱ������ڣ�����0.8 mol��H2��0.6 mol��I2����һ�������·������·�Ӧ��H2(g)+ I2(g)
I2(g) 2HI(g)
2HI(g)
��H<0����Ӧ�и����ʵ�Ũ����ʱ��仯�����ͼ1��
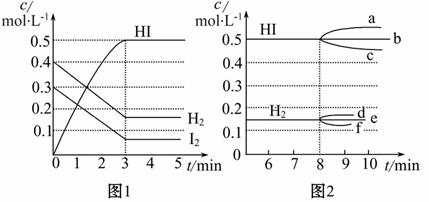
(1)�÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ__________��
(2)����ͼ1���ݣ���Ӧ��ʼ���ﵽƽ��ʱ��ƽ����Ӧ����v(HI)Ϊ_______��
(3)��Ӧ�ﵽƽ���8����ʱ��
���������¶ȣ���ѧƽ�ⳣ��K_________(�������С�����䡱)��HIŨ�ȵı仯��ȷ����___________(��ͼ2��a��c�ı�Żش�)��
��������I2��H2Ũ�ȵı仯��ȷ����_______(��ͼ2��d��f�ı�Żش�)��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��úת��Ϊˮú������Ҫ��ѧ��ӦΪC(s)��H2O(g)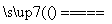 CO(g)��H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g)��H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
C(s)��O2(g)===CO2(g) ��H����393.5 kJ·mol��1
H2(g)��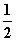 O2(g)===H2O(g) ��H����242.0 kJ·mol��1
O2(g)===H2O(g) ��H����242.0 kJ·mol��1
CO(g)��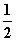 O2(g)===CO2(g) ��H����283.0 kJ·mol��1
O2(g)===CO2(g) ��H����283.0 kJ·mol��1
��ش�
(1)�����������ݣ�д��C(s)��ˮ������Ӧ���Ȼ�ѧ��Ӧ����ʽ��________________________________________________________________________��
(2)�ȽϷ�Ӧ�����ݿ�֪��1 mol CO(g)��1 mol H2(g)��ȫȼ�շų�������֮�ͱ�1 mol C(s)��ȫȼ�շų��������ࡣ��ͬѧ�ݴ���Ϊ��úת��Ϊˮú������ʹúȼ�շų����������������ͬѧ���ݸ�˹������������ѭ��ͼ��
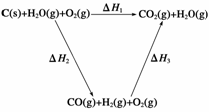
���ݴ���Ϊ��úת��Ϊˮú����ȼ�շų���������úֱ��ȼ�շų���������ȡ���
��������ס�����ͬѧ�۵���ȷ����__________(��ס����ҡ�)���жϵ�������________________________________________________________________________
________________________________________________________________________��
(3)��úת��Ϊˮú����Ϊȼ�Ϻ�úֱ��ȼ������кܶ��ŵ㣬���о����е������ŵ㣺________________________________________________________________________
________________________________________________________________________��
(4)ˮú������������������ȼ�ϣ�Ҳ����Ҫ���л�����ԭ�ϡ�CO��H2��һ�������¿��Ժϳɣ��ټ״����ڼ�ȩ���ۼ��ᡡ�����ᡣ�Է�����CO��H2��1��1������Ȼ�Ϸ�Ӧ���ϳ�����________(�����)����ʱ���������㡰��ɫ��ѧ����Ҫ����ȫ����ԭ���е�ԭ�ӣ�ʵ�����ŷš�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ͼ��ʾ�ķ�Ӧ�ж�����˵���д������( )
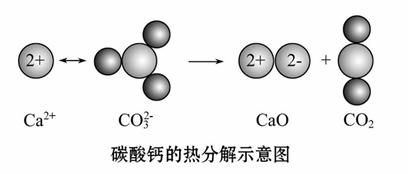
A��CO2(g)��CaO(s)������������CaCO3(s)��������
B���÷�Ӧ���ʱ������
C���÷�Ӧ�������Ӽ�����Ҳ�й��ۼ�����
D���ɸ÷�Ӧ���Ƴ�������Ҫ���Ȳŷ����ķ�Ӧ��Ϊ���ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڹ̶��ܱ������У����淴Ӧ2X(g)+2Y(s) 3Z(g)���ﵽƽ�������������X��Y��Z�����ʵ���������һ�룬�Ը÷�Ӧ������Ӱ����( )
3Z(g)���ﵽƽ�������������X��Y��Z�����ʵ���������һ�룬�Ը÷�Ӧ������Ӱ����( )
A�������淴Ӧ���ʶ���С��ƽ�ⲻ�ƶ�
B�������淴Ӧ���ʶ���С��ƽ��������Ӧ�����ƶ�
C������Ӧ���������淴Ӧ���ʼ�С��ƽ��������Ӧ�����ƶ�
D������Ӧ���ʼ�С���淴Ӧ��������ƽ�����淴Ӧ�����ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и����п�˵��HAc��������ʵ���( )
A.0.1 mol��L-1HAc��pH>1
B.��ͬ�¶��£�������ǿ����Һ�ĵ����ԱȽ�
C.��ͬ���ʵ���Ũ�ȡ���ͬ����Ĵ��������ֱ��������Ļ��ý�����Ӧʱ�����Ľ�������
D.pH=4�Ĵ�����pH=10��NaOH��Һ�������Ϻ���Һ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵ���Һ�����������ɡ����գ����ù���ijɷֲ���ͬ����
( )
A.FeCl2��FeCl3 B.FeCl2��Fe2(SO4)3
C.Na��Al(OH)4�ݡ�AlCl3 D.Mg(HCO3)2��MgCl2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ݱ��ж�����Ԫ�����ʵ������жϣ�����˵����ȷ����(����)
| Ԫ�ر�� Ԫ�����ʡ������� | �� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶/10��10 m | 0.66 | 1.36 | 1.23 | 1.10 | 0.99 | 1.54 | 0.70 | 1.18 |
| ������ ���ϼ� | ��2 | ��1 | ��5 | ��7 | ��1 | ��5 | ��3 | |
| ��2 | ��3 | ��1 | ��3 |
A.�٢��γɵĻ������������
B����λ�ڵڶ����ڵڢ�A��
C���ܢ��γɵĻ����������ӻ�����
D���۵�����������Ӧ��ˮ���������ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ��ȤС������Mn02��ŨHCl����ͼ��ʾװ���Ʊ�Cl2�����з����в���ȷ���ǣ�������
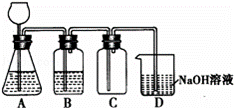
| �� | A�� | A�п��÷�Һ©�����泤��©�� |
| �� | B�� | A��ȱ�ټ���װ�� |
| �� | C�� | B��ʢ�ŵ�NaOH��Һ���Ծ���Cl2 |
| �� | D�� | D�еĵ��ܿ������ӵ���©���ɷ�ֹ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com