
| 134g |
| 134g/mol |
| 44.8L |
| 22.4L/mol |
| 33.6L |
| 22.4L/mol |
| 0.5mol��2 |
| 1mol |
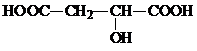 ��
��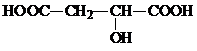 ��
��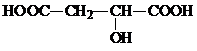 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ����ʮ���и߶���ѧ�����п������ƻ�ѧ�Ծ����������� ���ͣ������
��4�֣�ij�л���Ļ�ѧ���������¼���£�����Է�������Ϊ134����ȫȼ�պ�ֻ����CO2��ˮ����134g���л�����������NaHCO3��Ӧ�ų�44.8LCO2�����������л���������Na��Ӧ�ų�33.6L H2�����������������Ϊ��״�������۷�����ͬһ��̼ԭ���ϲ�������ͬ�Ĺ����š�
��1��д�����л������������ŵ����� �� ��
��2��д�����л���Ľṹ��ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ�߶���ѧ�����п������ƻ�ѧ�Ծ��������棩 ���ͣ������
��4�֣�ij�л���Ļ�ѧ���������¼���£�����Է�������Ϊ134����ȫȼ�պ�ֻ����CO2��ˮ����134g���л�����������NaHCO3��Ӧ�ų�44.8LCO2�����������л���������Na��Ӧ�ų�33.6L H2�����������������Ϊ��״�������۷�����ͬһ��̼ԭ���ϲ�������ͬ�Ĺ����š�
��1��д�����л������������ŵ����� �� ��
��2��д�����л���Ľṹ��ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ٸû�������Է�������Ϊ342
�ڷ�������ԭ������ԭ�Ӹ�����Ϊ2��1�����̼��10��
�۸�����������ˮ�����ܷ���������Ӧ
������ˮ��Һ�м�ϡH2SO4�������ȣ���Ӧ���õIJ�����Ժ����Ƶ�Cu(OH)2����Һ��Ӧ�����к�ɫ��������
������������ش��������⣺
(1)ͨ�����㣬�ɵó��û��������ʽ��______________��
(2)д���û�������ϡH2SO4���������·�����Ӧ�Ļ�ѧ����ʽ
_______________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com