
(16��)ij�о�С�����ô�ʳ��(��Ca2+��Mg2+��SO ��)���ֹ�(��C������Cl2��Ӧ�Ĺ�������)��ȡ���裬������µĹ������̣�
��)���ֹ�(��C������Cl2��Ӧ�Ĺ�������)��ȡ���裬������µĹ������̣�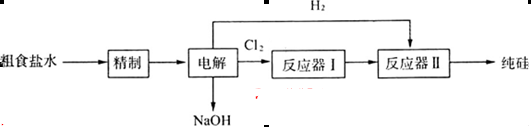
�Իش��������⣺
��1����ҵ��һ�������ù�����̿�ڸ����»�ԭʯӢɰ����ȡ�ֹ裬д���ù��̵Ļ�ѧ����ʽ��_______________________________________________________________________��
��2�����ƴ���ˮ�����Լ�Ϊ��BaC12����Na2CO3����HC1����NaOH����μӵ��Ⱥ�˳�������е�________(�����и�������)��
a���٢ڢܢ� b���ܢڢ٢� c���ܢ٢ۢ� d���ڢܢ٢�
��֪�� ������ô���ˮ��
������ô���ˮ�� ��Ũ�Ⱦ�Ϊ0.01 mol��L-1������1 L�ô���ˮ������һ����Na2CO3��Һ�����ȳ��ֵij�����__________��
��Ũ�Ⱦ�Ϊ0.01 mol��L-1������1 L�ô���ˮ������һ����Na2CO3��Һ�����ȳ��ֵij�����__________��
��3����֪SiCl4�ķе���57.6�棬CC14�ķе���76.8�档�ڷ�Ӧ��I�еõ���SiCl4��Ʒ�к���CCl4�����еõ�����SiCl4�ɲ��õķ��������и����е�________(�����)��
a������ b������ c����Һ d������
��Ӧ�����з�����Ӧ�Ļ�ѧ����ʽΪ__________________________________________��
��4����ͼ�������ӽ���Ĥ����ⱥ��ʳ��ˮ��ʾ��ͼ������������������������_____����ƷA�Ļ�ѧʽΪ____________��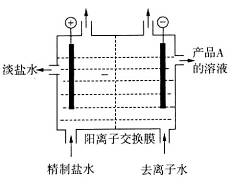
��������Ĥ���۵�ⱥ��ʳ��ˮ����ȡ�������ƣ���д���÷�Ӧ�Ļ�ѧ����ʽ__ ___��
��16�֣�
��1��SiO2+2C Si+2CO����2�֣�
Si+2CO����2�֣�
��2��a��2�֣���̼��ƣ���CaCO3����2�֣�
��3��a��2�֣���SiCl4+2H2 Si+4HCl��2�֣�
Si+4HCl��2�֣�
��4����������H2����2�֣���NaOH��2�֣���NaCl+H2O  NaClO+H2����2�֣�
NaClO+H2����2�֣�
���������������1��C�ڸ��������»�ԭSiO2������Si��CO����ѧ����ʽΪ��SiO2+2C Si+2CO��
Si+2CO��
��2��Na2CO3��Һ������Ϊ��ȥCa2+����ȥ������BaCl2��Һ������Na2CO3��˳����BaCl2�ĺ��棬HCl�������dz�ȥ������Na2CO3��NaOH��������������a����ȷ����ΪKsp(MgCO3) < Ksp(CaCO3)��CaCO3�����ܣ��������������ij�����CaCO3��
��3��SiCl4��CCl4�ڳ�����ΪҺ�壬��ܽ⣬���е㲻ͬ������������ķ����õ�������SiCl4����Ӧ��II��H2��ԭSiCl4����ѧ����ʽΪ��SiCl4+2H2 Si+4HCl��
Si+4HCl��
��4�����ݷŵ�˳��������H2O�������H+�ŵ磬���Ե���������������������������ˮ�������H+�ŵ磬�ٽ�H2O�ĵ���ƽ�������ƶ���OH?Ũ���������Բ�ƷAΪNaOH����Ĥ���۵�ⱥ��ʳ��ˮ����ȡ�������ƣ���������Cl2��NaOH��Ӧ���ɴ������ƣ����Ի�ѧ����ʽΪ��NaCl+H2O  NaClO+H2��
NaClO+H2��
���㣺���⿼�黯ѧ����ʽ����д�����ӡ�����Ӧ�á�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ�Ƿḻ����Դ���⣬�Ӻ�ˮ����ȡԪ���ǻ�ѧ��ҵ����Ҫ��ɲ��֡�
��1�����ξ��ƾ��dz�ȥ���е�Ca2+��Fe3+��SO42-����ɳ�����ʣ��������Լ��У���Na2CO3��Һ ��HCl�����ᣩ ��Ba��OH��2��Һ���������Լ�������˳����_________������ţ���
��2�����������С���ˮ���塱����ȡ�嵥�ʷ�Ӧ�����ӷ���ʽΪ��__________��
��3��ijͬѧ�������ͼװ�ý������µ绯ѧʵ�顣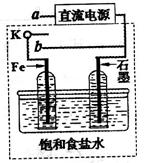
�ٵ�����K��a����ʱ�������������ݲ�����������Ϊ_______����
��һ��ʱ���ʹ����K��a�Ͽ�����b����ʱ�����߿��ڵ�װ�ÿɳ�Ϊ__________����д����ʱFe�缫�ϵĵ缫��Ӧʽ_________________��
��4��ij������ʢ��CaSO4����Һ�ķ�Ӧ����ͨ�백������ȡ���ʣ�NH4��2SO4��Ч�����á���ͨ��CO2��������������NH4��2SO4���������ԭ�� ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
����ʹ�ò��ϣ������������
�� ���������������������зdz���Ҫ�IJ��ϣ���ÿ������ʴ����ʧ������ʮ�־��ˡ�������ʴ��Ϊ��ѧ��ʴ�͵绯ѧ��ʴ�����ߵ��۱��ʶ��Ƿ��� �Ĺ��̡�Ϊ��ֹ�ִ������ں�ˮ�б���ʴ��һ���ڴ������� ��ѡ�п�顱��ͭ�顱����
�� ����ͨ������������У�̼�������������ڸ����·�����Ӧ�Ļ�ѧ����ʽ��
������������̥����Ҫԭ�ϣ���Ȼ��ͨ����ʩ��ʹ���ķ���ת��Ϊ �ṹ���Ӷ�������ǿ�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
�����̿�MnCO3��������Fe2O3��FeO��HgCO3��2HgO�����ʣ���ҵ�������̿���ȡ�̣������������£�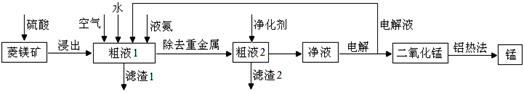
��ش��������⣺
��1�����Һ1�м����ˮ�����Ҫ �������ܴﵽ����Ҫ��
��2���������õĿ�������Ĥ���뷨�Ʊ��ĸ����������÷�����ԭ���� ��
��3����������Ҫ�ɷ�Ϊ(NH4)2S����Һ2�з�����Ҫ��Ӧ�����ӷ���ʽΪ ��
��4��д�������ĵ缫��Ӧʽ ��˵�����Һѭ����ԭ�� ��
��5��д�����ȷ����̵Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��17�֣���ѧʵ�����������⻯ѧ֪ʶ���γɻ�ѧ������̽���봴��������������ѧ������
��1����ʵ��������Ũ������MnO2������ȡCl2���������ʵ�顣
�������ռ�Cl2����ȷװ���� ��
�ڽ�Cl2ͨ��ˮ�У�������Һ�о��������Եĺ��������� ��
�����ʵ��Ƚ�Cl2��Br2�������ԣ������������ǣ�ȡ����������ˮ��CCl4���Թ��У� ��
��2������֮������ת�������ʳ��ˮ�Ʊ�Cl2�ǽ�����ת��Ϊ��ѧ�ܣ���ԭ��ؿɽ���ѧ��ת��Ϊ���ܡ�����������͵�ԭ��أ�̽��������ת��Ч�ʡ�
��ѡ���ϣ�ZnSO4(aq)��FeSO4(aq)��CuSO4(aq)��ͭƬ����Ƭ��пƬ�͵��ߡ�
�����ԭ��ؼ�װ��ʾ��ͼ��������Ӧ��ע��
Ҫ����ͬһ�ձ��У��缫����Һ����ͬ�Ľ���Ԫ�ء�
��ͭƬΪ�缫֮һ��CuSO4(aq)Ϊ�������Һ��ֻ��һ���ձ�����װԭ����ң�����һ��ʱ��ɹ۲쵽���� ��
�ۼ�������ԭ����пɸ���Ч�ؽ���ѧ��ת��Ϊ���ܵ��� ����ԭ���� ��
��3��������������������������ԭ����Ϊ�����������Һ����Ƭ�ĸ�ʴ���ڣ�2���IJ�����Ӧѡ
��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС���������ͼ��ʾװ�õ�ⱥ���Ȼ�����Һ�Ʊ�H2��ͨ��H2��ԭ����ͭ�ⶨCu�����ԭ������Ar��Cu����ͬʱ����Cl2�������ԣ�ͼ�мгֺͼ�����������ȥ����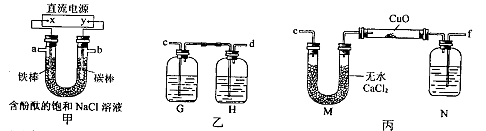
��1��ֱ����Դ�е�X��Ϊ �����������������������������������д����װ��U�ι��з�Ӧ�����ӷ���ʽ�� ��ʵ�鿪ʼ�����������缫��һ���ʵ�������� ��
��2��Ϊ�������ʵ�飬��ȷ������˳��Ϊ��a�� ��b�� ����д���ӵ���ĸ����
��3��װ�����е�Gƿ����Һ����Ϊ ������ĸ����
| A������KI��Һ | B��NaOH��Һ | C��Na2S��Һ | D��Na2SO3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����й�ͬ���칹����Ŀ�������У�����ȷ����( )
| A���ױ������ϵ�һ����ԭ�ӱ���3��̼ԭ�ӵ����ȡ�������ò�����6�� |
| B������ʽ����C5H11Cl�Ļ�������6�� |
| C����֪���ȱ���3��ͬ���칹�壬�����ȱ���ͬ���칹�����ĿΪ3�� |
D���ƵĽṹ��ʽΪ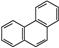 ���������ᷴӦ��������5��һ����ȡ���� ���������ᷴӦ��������5��һ����ȡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�±���ʵ������ܴﵽʵ��Ŀ�ĵ���
| | ʵ����� | ʵ��Ŀ�� |
| A | �ӵı�����Һ�еμ�ϡ��ˮ | ��֤���屽��Ϊ��ɫ���� |
| B | ���������Һ�м����Ƶ�Cu(OH)2����Һ������ | ȷ���������к���ȩ�� |
| C | ��ƾ�������Ļ��Һ�м�������� | ȷ���ƾ��л��д��� |
| D | ��������������������Һ����һ��ʱ�䣬������ȴ��Ļ��Һ�еμ���������Һ | ����ˮ������е������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�±������еĶ������������ǵ�һ±ȡ�����ֻ��һ�֣������±��и�����Ų����ɣ����˹����Ų���6��ӦΪ�� ��
| 1 | 2 | 3 | 4 | 5 | ���� |
| CH4 | C2H6 | C5H12 | C8H18 | ���� | ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com