


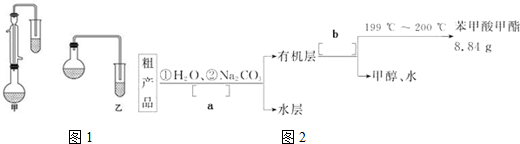
���� ��1����װ�â��з����ķ�Ӧ��֪��װ�â��в���������ΪSO2���������������ᷴӦ���������ơ�����������ˮ��
��2��װ�â��л���������ľ��壬���������Һ̬��Ӧ��ȡ���˲�����
��3��װ�â����ڴ���β��������Ϊ��Ӧ�Ķ�������Ӧ��ֹ�������Ҳ��ܴ�����ȫ�ܱջ����У�
��4��NaHSO3��Һ��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ���Һ�����ԣ��ʼ�����Һ�����Լ��ɣ�
��5��Na2S2O5�����ڿ������ױ�����ΪNa2SO4�������ᡢ�Ȼ�����Һ������Ʒ���Ƿ�����������ɣ�
��6���������ĵ���������SO2+I2+2H2O�TH2SO4+2HI��������������������������Ũ�ȣ�
�����в���HI�����������������ĵ����ƫС���ʲⶨ�����������ƫС��
��� �⣺��1����װ�â��з����ķ�Ӧ��֪��װ�â��в���������ΪSO2���������������ᷴӦ���������ơ�����������ˮ����Ӧ����ʽΪ��Na2SO3+H2SO4=Na2SO4+SO2��+H2O��
�ʴ�Ϊ��Na2SO3+H2SO4=Na2SO4+SO2��+H2O��
��2��װ�â��л���������ľ��壬���������Һ̬��Ӧ��ȡ���˽��з��룬
�ʴ�Ϊ�����ˣ�
��3��a��װ��Ӧ���������백ˮ�п������ն�������Ϊ�ܱջ�����װ����ѹǿ�����ײ�����ȫ�¹ʣ��ʴ���
b����װ�����ն������������ϲ��Ϊ�ܱջ�����װ����ѹǿ�����ײ�����ȫ�¹ʣ��ʴ���
c����װ�ò������ն�������������ʵ��ʵ��Ŀ�ģ��ʴ���
d����װ���������������������Ӧ���������գ��ҷ�ֹ����������ȷ��
�ʴ�Ϊ��d��
��4��NaHSO3��Һ��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ���Һ�����ԣ��ⶨ��Һ��pH������ȷ����Һ����ԣ�������Һ����ʹʪ����ɫʯ����ֽ��죬�������òⶨ��ҺpHֵ��ʪ�����ɫʯ����Һ���飬������Ba��OH��2��Һ��HCl��Һ��Ʒ����Һ������˵����Һ�����ԣ���ѡae��
�ʴ�Ϊ��ae��
��5��Na2S2O5��SԪ�صĻ��ϼ�Ϊ+4�ۣ���˻ᱻ����ΪΪ+6�ۣ��������ڿ������ױ�����ΪNa2SO4�������ᡢ�Ȼ�����Һ������Ʒ���Ƿ�����������ɣ�ʵ�鷽��Ϊ��ȡ����Na2S2O5�������Թ��У���������ˮ�ܽ⣬�μ����ᣬ���ٵμ��Ȼ�����Һ���а�ɫ�������ɣ�
�ʴ�Ϊ��ȡ����Na2S2O5�������Թ��У���������ˮ�ܽ⣬�μ����ᣬ���ٵμ��Ȼ�����Һ���а�ɫ�������ɣ�
��6������100mL���Ѿ��ж������������Ϊmg����
SO2+2H2O+I2�TH2SO4+2HI
64g 1mol
mg 0.025L��0.01mol/L
���ԣ�64g��mg=1mol��0.025L��0.01mol/L��
���m=0.016
�ʸô�ʵ������Ʒ�п��������IJ�������������SO2���㣩Ϊ$\frac{0.016g}{0.1L}$=0.16 g/L
�ʴ�Ϊ��0.16��
�����в���HI�����������������ĵ����ƫС���ʲⶨ�����������ƫС����ⶨ���ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
���� ���⿼�����ʵ��Ʊ�ʵ�顢ʵ�鷽����ơ����ʺ����IJⶨ��������ԭ��Ӧ�ζ��ȣ��Ѷ��еȣ���ȷʵ��ԭ���ǽⱾ��ؼ����������ʵ����ʷ������ע��Ԫ�ػ�����֪ʶ�Ļ��ۺ�������ã�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �¶� | 25�� | 50�� | 95�� |
| �ܽ�� | 0.17g | 0.95g | 6.8g |
| ���� | �״� | ������ | ��������� |
| ��Է������� | 34 | 122 | 136 |
| �е�/�� | 64.7 | 249 | 199.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

| �� �� | �� �� | �� �� | ԭ �� |
| Na2S2O4Ϊǿ�������Σ�����ҺΪ���ԣ� | ȡ������Һ���Թ��У��μ� ����ɫʯ����Һ | ��Һ�����ɫ | S2O42-ˮ�⣬ʹ��Һ�ɼ��� |
| ��Na2S2O4���л�ԭ�� | ȡ������Һ���Թ��У��μӹ���������ˮ���ٵμ� BaCl2 ��Һ | �а�ɫ�������� | �÷�Ӧ�����ӷ���ʽ����Ϊ����4H2O+S2O42-+3Cl2=2SO42-+6Cl-+8H+����Ba2++SO42-=BaSO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
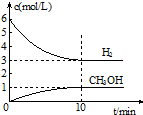 T��ʱ����1L���ܱ������г���2mol CO2��6mol H2��������Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ•mol-1�����H2��CH3OH��g����Ũ����ʱ��仯�����ͼ��ʾ������˵������ȷ���ǣ�������
T��ʱ����1L���ܱ������г���2mol CO2��6mol H2��������Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ•mol-1�����H2��CH3OH��g����Ũ����ʱ��仯�����ͼ��ʾ������˵������ȷ���ǣ�������| A�� | 0��10min��v��H2��=0.3 mol•L-1•min-1 | |
| B�� | T��ʱ��ƽ�ⳣ��K=$\frac{1}{27}$��CO2��H2��ת������� | |
| C�� | T��ʱ������32 g CH3OH����ʱ���ų�49.0 kJ������ | |
| D�� | �ﵽƽ��������¶Ȼ��ٳ���CO2���壬���������H2��ת���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ζ����� | ���ⰱˮ��Һ�����/mL | 0.10mol/L��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com