
����Ŀ��������Ҫ�ĺϽ�Ԫ�أ������������������ȺϽ���Ҳ�������л�����Ĵ�������ȡ����
��1��д����̬Cr�ļ����Ų�ʽ__________��Cr�й���__________�ֲ�ͬ�ܼ��ĵ��ӡ�
��2��Ni(CO)n��Fe(CO)5ͬ�������ʻ������γ������ʱ��ÿ��CO�ṩһ�Ե��������ԭ���γ���λ�����о����ֽ���ԭ�ӵļ۵��Ӻ�CO�ṩ�ĵ����ܺ͵���18��
��Ni��C��O�ĵ縺���ɴ�С��˳��Ϊ____________________��
�� Ni(CO)n������n=__________��
����֪Ni2+��Fe2+�����Ӱ뾶�ֱ�Ϊ69pm��78pm�������ҽ������������ڵ�NiO��FeO�ҽ�����ȴ�����У�NiO�������Ƚᾧ���Խ�����ԭ��____________________��
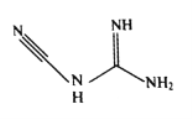
��3���춨�����ܡ�ͭ�ٵȿ���˫�谷����ѧʽC2H4N4����ṹ��ʽ��ͼ��ʾ��˫�谷������̼ԭ�ӵ��ӻ���ʽ��__________�����ӽṹ�м������Ĺ��ۼ���__________��
��4�����ľ���ṹ�������Ͻ�ľ�����ͼ��ʾ��
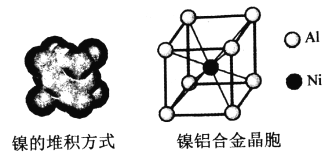
��������Ķѻ���ʽΪ__________��
����֪Al�ĵ�һ���ڶ������ֱܷ�Ϊ��l1=578kJ/mol��l2=1817kJ/mol������l2Զ����l1��ԭ��__________________________________________________��
����֪�������Ͻ��ܶ�Ϊdg/cm3��NA��������٤���������������ĺ˼��Ϊ__________ pm��(�ô���ʽ��ʾ)
���𰸡� [Ar]3d3 6 O>C>Ni 4 Ni2+���Ӱ뾶С��Fe2+��NiO�����Ӽ���ǿ�������ܸ��ߣ���NiO�۵����FeO,�ҽ���ȴ������NiO�Ⱦ��� sp��sp2 C![]() N �����������ܶѻ���ccp��fcc ��ΪAlʧȥһ�����Ӻ��������3s2,s��ȫ����״̬���Ƚ��ȶ����ڶ������Ӻ���ʧȥ������I2Զ����I1
N �����������ܶѻ���ccp��fcc ��ΪAlʧȥһ�����Ӻ��������3s2,s��ȫ����״̬���Ƚ��ȶ����ڶ������Ӻ���ʧȥ������I2Զ����I1 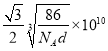
����������1��Cr����24��Ԫ�������ݹ���ԭ����֪Cr�ĺ�������Ų�ʽ��1s22s22p63s23p63d3����Ϊ[Ar]3d3���ɻ�̬��������Ų�ʽ��֪����6�ֲ�ͬ�ܼ���
��2����Ni��C��O�ĵ縺���ɴ�С��˳��ΪO>C>Ni���� Ni(CO)n�����н���ԭ�ӵļ۵��Ӻ�CO�ṩ�ĵ����ܺ͵���18��Ni�ļ۵�����Ϊ10��һ��CO�ṩ2�����ӣ�����nӦ����4����Ni2+���Ӱ뾶С��Fe2+��NiO�����Ӽ���ǿ�������ܸ��ߣ���NiO�۵����FeO,�ҽ���ȴ������NiO�Ⱦ�����
��3�����ݽṹ��ʽͼ��Cԭ�ӵĻ�ѧ�����ӷ�ʽ��ֱ���ͺ�ƽ�����������ֹ��ͣ��ɴ��ƶ��ӻ���ʽΪsp��sp2���֣����ܴ�С˳��Ϊ������>˫��>�������ʷ��ӽṹ�м������Ĺ��ۼ���C![]() N��
N��
��4���ٸ���ͼʾ��֪��������Ķѻ���ʽΪ�������������ܶѻ���ccp��fcc����Al�Ļ�̬�����Ų�ʽΪ1s22s22p63s23p1��ʧȥ3p1�ĵ��ӱȽ��������Ե�һ�����ܽ�С��ʧȥ3p1��3s2Ϊȫ����������ʧȥ���ӣ����Եڶ�������Զ���ڵ�һ�����ܡ��۸��ݾ����ṹ��һ�������к���1��Alһ��Ni������һ������������Ϊ�� ![]() g�������ı߳�Ϊ
g�������ı߳�Ϊ![]() cm���������ľ���Ϊ�����Խ��ߵ�һ�룬���Ծ���Ϊ
cm���������ľ���Ϊ�����Խ��ߵ�һ�룬���Ծ���Ϊ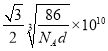 pm��
pm��


 ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��![]() ��һ���л��ϳɵ��м��壬����ͨ������;�����ϳɣ�
��һ���л��ϳɵ��м��壬����ͨ������;�����ϳɣ�

��֪��(1) 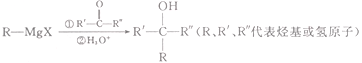
(2) 
(3) ![]()
�ش��������⣺
(1)M�ķ���ʽΪ__________________��
(2)N��ϵͳ������������Ϊ_____________________��
(3)D�й���������Ϊ__________________��
(4)��A����B�Ļ�ѧ����ʽΪ___________________����Ӧ����Ϊ________________��
(5)H�DZ�G��6��̼ԭ�ӵ�G��ͬϵ���H��_________�֣����к˴Ź����������������շ�Ľṹ��ʽΪ___________________��
(6)д�����ȱ�Ϊԭ���Ʊ������� �ĺϳ�·�ߣ�______________________��(�����Լ���ѡ)��
�ĺϳ�·�ߣ�______________________��(�����Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ������ʾ��װ���У��ձ���ʢ�ŵ���Ba(OH)2��Һ�����ӵζ���������ij����ҺAʱ����Һ�ĵ����Եı仯������ͼ������ʾ��


(1)�μ�Һ����ͼ������������͵�ʱ�����ݿ���Ϩ�𣬿��ܵ�ԭ����__________________��
(2)�Ը������ӷ�Ӧ���ص��������ҺA�к��е����ʿ����ǣ�����ţ�____________��
��HCl ��H2SO4 ��NaHSO4 ��NaHCO3
(3)��֪0.1 mol��L-1NaHSO4��Һ��c(H��)=0.1 mol��L-1����ش��������⣺
��д��NaHSO4��ˮ��Һ�еĵ��뷽��ʽ___________________________��
��NaHSO4����________������������������������������
����NaHSO4��Һ�У���μ���Ba(OH)2��Һ�����ԣ���д��������Ӧ�����ӷ���ʽ��____________��������������Һ�У������μ�Ba(OH)2��Һ����д���˲���Ӧ�����ӷ���ʽ��_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����50 mL NaOH��Һ����ͨ��һ������CO2(������Һ�������)�����ȡ����Һ10 mL������ϡ����100 mL�������ϡ�ͺ����Һ����μ���0.1 mol��L-1���ᣬ����CO2��������(��״����)������������������ϵ����ͼ��ʾ��

(1) д��OA����������Ӧ�����ӷ���ʽ��______________��
(2)NaOH������CO2��������Һ������Ϊ____�������ʵ���Ũ��֮��Ϊ____��
(3)����CO2�����(��״����)Ϊ____��
(4)ԭNaOH��Һ�����ʵ���Ũ��Ϊ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��W��X��Y��Z��Q��ԭ��������������c��d��e��f��h������ЩԪ����ɵĶ�Ԫ�������Ȼ����Ӳ�����ĵ��ʺ�a����X��ɣ�b��W��Y��Q����Ԫ�����0.05mol/Lb��Һ��pHΪ1��d��ʹƷ����Һ��ɫ��e��Һ�壬f����ɫ��ӦΪ��ɫ���������ʵ�ת����ϵ��ͼ��ʾ(���������ȥ)������˵��������ǣ� ��

A. ��Ԫ������е㣺e>d>c B. �⻯����ȶ��ԣ�Q>Y>X
C. Ԫ�صķǽ����ԣ�Y>X>W D. ԭ�Ӱ뾶�Ĵ�С��Z>Q>Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������£��������ʿ��Է����Ӿ۷�Ӧ���ɸ߷��ӻ��������
A.����B.����C.�Ҵ�D.��ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и������ܴ������棬��������Ӧ�Լ���ᷢ����ѧ�仯���ҷ�����Ӧ�����ӷ���ʽ��д��ȷ����( )
ѡ�� | ���飨ˮ��Һ�� | �����Լ� | ���ӷ���ʽ |
A | H+�� Na���� | Fe�� | Fe+H+=Fe3++H2�� |
B | Na����Cl���� | ������ | 2Na+2H2O=2Na��+2OH-+H2�� |
C | NH4+��H+��CH3COO- | ������ | 6H++Fe2O3=2Fe3++3H2O |
D | Ca2����OH-��Cl�� | ͨ�����CO2 | OH-+CO2= |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ϳ���(CO��H2)��Ŀǰ�������õ�ԭ�ϣ��������ü����Ʊ��ϳ��������ַ�����
�� CH4(g)+H2O(g)![]() CO(g)+3H2(g) ��H1=+216 kJ��mol-1��
CO(g)+3H2(g) ��H1=+216 kJ��mol-1��
�� 2CH4(g)+O2(g) ![]() 2CO(g)+4H2(g) ��H2=-72 kJ��mol-1��
2CO(g)+4H2(g) ��H2=-72 kJ��mol-1��
����һ����Ӧ�ķ�Ӧ�����������仯��ϵ��ͼ��ʾ��������˵����ȷ������ ��

A. E1��ʾ2CH4(g)+O2(g) ![]() 2CO(g)+4H2(g)�Ļ��
2CO(g)+4H2(g)�Ļ��
B. E2��ʾCH4(g)+H2O(g)![]() CO(g)+3H2(g)�Ļ��
CO(g)+3H2(g)�Ļ��
C. ��ͼʾΪ��Ӧ���ķ�Ӧ�����������仯ʾ��ͼ
D. һ������£�������������ܽ���E1��Ҳ�ܽ���E2�������ܸı�E1��E2�IJ�ֵ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������£�CO2(g)+3H2(g)![]() CH3OH (g)+H2O(g) ��H=��57.3 kJ/mol���� 2L �����ܱ������г��� 1 mol CO2��3 mol H2���ڲ�ͬ���������·�����Ӧ�١���Ӧ���뷴Ӧ�ۣ���ͬʱ����CO2��ת�������¶ȱ仯����ͼ��ʾ��b�㷴Ӧ�ﵽƽ��״̬������˵����ȷ����
CH3OH (g)+H2O(g) ��H=��57.3 kJ/mol���� 2L �����ܱ������г��� 1 mol CO2��3 mol H2���ڲ�ͬ���������·�����Ӧ�١���Ӧ���뷴Ӧ�ۣ���ͬʱ����CO2��ת�������¶ȱ仯����ͼ��ʾ��b�㷴Ӧ�ﵽƽ��״̬������˵����ȷ����
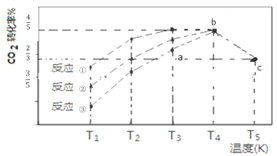
A. a �� v(��)>v(�棩
B. b�㷴Ӧ����53.7 kJ
C. ����Ч����ѵķ�Ӧ�Ǣ�
D. c��ʱ�÷�Ӧ��ƽ�ⳣ��K=4/3(mol-2![]() L-2)
L-2)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com