
| �Ҵ� | ������ | �� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ���ɫҺ�� |
| �ܶ�/��g•cm-3�� | 0.79 | 1.44 | 3.1 |
| �е�/�� | 78.5 | 38.4 | 59 |
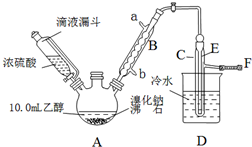
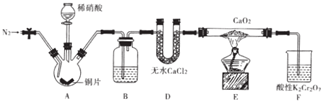
���� ��1������װ��ͼ��֪A���������ƣ�
��2����Һ©������ʹ©�����Ϸ����·���ѹǿ��ȣ�ʹŨ����˳�����£�
��3�����¶ȹ���Ũ�����Ũ�ȹ��������£�Ũ�������廯�ⷢ��������ԭ��Ӧ������Ͷ�������ʹ�ռ����Ĵֲ�Ʒ�ʳ�ɫ��
��4����A���ȿ��Լӿ췴Ӧ�������������飻ʵ���в����Ķ��������廯�⡢��ȿ�����ϡNaOH��Һ���գ�
��5�������������������ʣ���һ�����ܹ����廯�ⷴӦ�Ҳ��ܺ������鷴Ӧ�Լ���ע����Ӳ��������µ����ʣ�
��6������������ܶȴ���ˮ�����Է�Һ���Ǵӷ�Һ©�����¿ڷų���
��10mL�Ҵ�������Ϊ0.79��10g=7.9g�������ʵ���Ϊ0.172mol�����������Ƶ�����������ʵ���Ϊ0.172mol��������Ϊ18.75g�����ݲ���=$\frac{ʵ�ʲ���}{���۲���}$��100%���㣮
��� �⣺��1������װ��ͼ��֪A����������Ϊ������ƿ��
�ʴ�Ϊ��������ƿ��
��2����Һ©������ʹ©�����Ϸ����·���ѹǿ��ȣ�ʹŨ����˳�����£�����Һ©��û��������ܣ�
�ʴ�Ϊ��ƽ��ѹǿ��ʹŨ����˳�����£�
��3�����¶ȹ���Ũ�����Ũ�ȹ��������£�Ũ�������廯�ⷢ��������ԭ��Ӧ������Ͷ�������ʹ�ռ����Ĵֲ�Ʒ�ʳ�ɫ����Ӧ����ʽΪ2HBr+H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$Br2��+SO2��+2H2O��
�ʴ�Ϊ��2HBr+H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$Br2��+SO2��+2H2O��
��4�����ȵ�Ŀ���Ǽӿ췴Ӧ���ʣ��¶ȸ���38.4��������ȫ���ӷ����������ʵ���в����Ķ��������廯�⡢��Ȼ���Ⱦ���������Կ�����ϡNaOH��Һ���գ�
�ʴ�Ϊ�������¶ȼӿ췴Ӧ���ʣ�ͬʱʹ���ɵ�������������������ٽ�ƽ���ƶ�������SO2��Br2��HBr��ֹ������Ⱦ��
��5��A���������ƣ����������ƻ�����������ˮ�⣬��A����
B����ȥ�������е���������Br2���ӵ⻯�ػ�����ⵥ������ ��B����
C������������ֻ���巴Ӧ���������鷴Ӧ����C��ȷ��
D��̼��������Һ�ʼ��ԣ����嵥�ʡ������鷴Ӧ����D����
��ѡC��
��6������������ܶȴ���ˮ�����Է�Һ���Ǵӷ�Һ©�����¿ڷų���
�ʴ�Ϊ���¿ڣ�
��10mL�Ҵ�������Ϊ0.79��10g=7.9g�������ʵ���Ϊ0.172mol�����������Ƶ�����������ʵ���Ϊ0.172mol��������Ϊ18.75g������������IJ���=$\frac{10.0g}{18.75g}$��100%=53.3%��
�ʴ�Ϊ��53.3%��
���� ���⿼���Ʊ�ʵ�鷽����ƣ�Ϊ��Ƶ���㣬�漰���㡢����������������ԭ��Ӧ�����ӵ�֪ʶ�㣬��ȷʵ��ԭ�����������ʡ����������淶���ǽⱾ��ؼ���֪������װ�ÿ��ܷ����ķ�Ӧ����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ϊ18����ԭ�ӣ�${\;}_{16}^{34}$S | |
| B�� | �����ӵĵ���ʽ��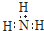 | |
| C�� | �������ƵĽṹ��ʽ��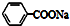 | |
| D�� | H2SO3�ĵ��뷽��ʽ��H2SO3?2H++SO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �Ķ�����ʵ�����ݣ�������ĿҪ��ش����⣮
�Ķ�����ʵ�����ݣ�������ĿҪ��ش����⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
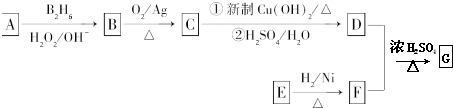
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ�� �����Һ | A | B | C | D | E | F |
| 4mol/L H2SO4/mL | 30 | V1 | V2 | V3 | V4 | V5 |
| ����CuSO4��Һ/mL | 0 | 0.5 | 2.5 | 5 | V6 | 20 |
| H2O/mL | V7 | V8 | V9 | V10 | 10 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʳ�ð��ǵ���Ҫ�ɷ��������� | |
| B�� | �ԡ��ع��͡����з���ɵõ����� | |
| C�� | NH3��Һ������������� | |
| D�� | �����ֺ�����������Ա��������������ˮ��������ɱ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | IA | ��A | ��A | ��A | VA | VIA | V��A | 0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | �� | ||
| 4 | �� | �� |
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
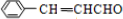 ��
��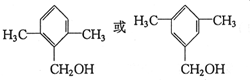 ��
��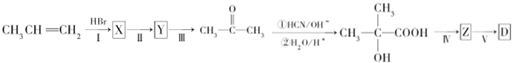
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HBr��HCl��HF | B�� | BaSO4��NH4Cl��CH3COONa | ||
| C�� | NaOH��Ca��OH��2��NH3•H2O | D�� | HClO��CaCl2��SO2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com