��2012?��ɽ����ģ��һѧϰС��������ͼ��ʾװ�ã���ij������Fe�ķ�ͭм����ͭ�����IJⶨ����̽���������Ʊ�����ͭ��Һ��
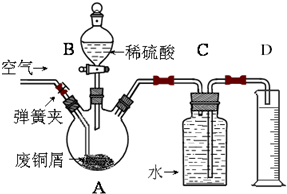
��1����A�м���10g��ͭм��Ʒ���رյ��ɼУ���B������Aע������ϡ�����رգ���ʱװ��C�з�����������
ˮ���½���ˮ˳����������D��
ˮ���½���ˮ˳����������D��
��
��2����Ӧ���е�A�в��ٲ�������ʱ����C���ռ���448mL���ѻ���ɱ�״�������壬��÷�ͭм��ͭ�������ٷֺ���Ϊ
88.8%
88.8%
��
��3�����C��Dװ�ú��ɼУ�����������ͨ��A�У���A�й���ȫ����ʧ���ټ���ͨ��һ��ʱ������رյ��ɼУ�ֹͣͨ�������
�ٸù����з�����Ӧ�����ӷ���ʽ��
4Fe2++O2+4H+=4Fe3++2H2O
4Fe2++O2+4H+=4Fe3++2H2O
��
2Fe3++Cu=2Fe2++Cu2+
2Fe3++Cu=2Fe2++Cu2+
��
��ΪʹA�й���ӿ��ܽ����ʣ����·������ú�������
abde
abde
��
a����Aװ�ü��� b����A�ڼ�������Fe
2O
3 c����A�ڼ�������CuO
d���������ͨ���� e����A�ڼ�������FeSO
4 f����A�ڼ�������H
2O
��4����A����Һ�����ձ��ڣ�����Cu
2��OH��
2CO
3���������pH=4ʱ����Һ��������ȫ���������˺ú��ɫ����������ͭ��Һ���˹������ӷ�Ӧ����ʽ��
Cu2��OH��2CO3+H++Fe3+=2Cu2++Fe��OH��3��+CO2��
Cu2��OH��2CO3+H++Fe3+=2Cu2++Fe��OH��3��+CO2��
��
��5����ͬѧ��Ϊ�����Բ��ò������巨�����ͭм��ͭ�������ٷֺ�����������õķ�����
�ⶨ������
�ⶨ������
�����������������дΪ��
����4�������ú��ɫ����ϴ�ӡ��������������
����4�������ú��ɫ����ϴ�ӡ��������������
��





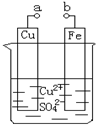 ��2012?��ɽ����ģ��ijС��Ϊ�о��绯ѧԭ���������ͼװ�ã�������������ȷ���ǣ�������
��2012?��ɽ����ģ��ijС��Ϊ�о��绯ѧԭ���������ͼװ�ã�������������ȷ���ǣ�������