

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | �Ҷ��� | �Ҷ��ᾧ�� |
| ����ʽ | H2C2O4 | H2C2O4?2H2O |
| ��ɫ״̬ | ��ɫ���� | ��ɫ���� |
| �ܽ�ȣ�g�� | 8.6��20�棩 | - |
| �۵㣨�棩 | 189.5 | 101.5 |
| �ܶȣ�g?cm-3�� | 1.900 | 1.650 |
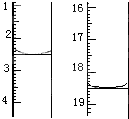
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
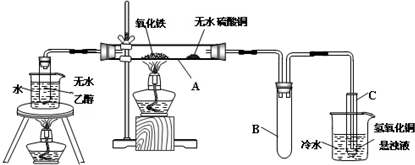
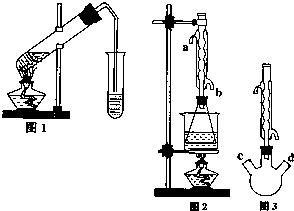 ������������Ҫ�Ĺ�ҵԭ�Ϻ��ܼ���ͨ�����������Ҵ������ᣮijѧϰС������ͼ1װ����ȡ���������ֲ�Ʒ���ٷ������������ĺ�����
������������Ҫ�Ĺ�ҵԭ�Ϻ��ܼ���ͨ�����������Ҵ������ᣮijѧϰС������ͼ1װ����ȡ���������ֲ�Ʒ���ٷ������������ĺ�����| �������� | �Ҵ� | ���� | |
| �е� | 77.1�� | 78.5�� | 117.9�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
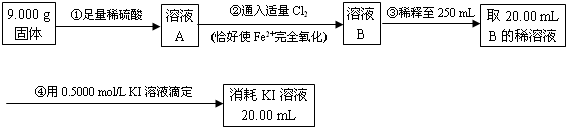
��15�֣�ij�о���ѧϰС��������ͼ��ʾװ���о��Ҵ����������ķ�Ӧ����ش��������⣺
��1��װ�����Թ�B�������� ��
��2��ʵ���пɹ۲쵽ʯӢ��A�е�����Ϊ ��
��3����Ӧֹͣ��ȡ���Թ�C�ھƾ����ϼ��������ڣ��ɹ۲쵽�к�ɫ����������д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4��Ϊ�˲ⶨ��Ӧ��ʯӢ��A����������Ԫ�صĺ�������������ʵ�飺
��i�� ��������õ��IJ����������ձ�������������ͷ�ιܡ� ��
��ii�������йز���ܵIJ�����˵����ȷ���� ��
a���ζ������п����õ�����Һ��Ϊָʾ��
b���ζ���������ˮϴ�Ӻ����ֱ��װҺ
c����ƿ����Ҫ�ô���ҹ��ϴ
d���ζ������У��۾�ע�ӵζ�����Һ��仯
e���ζ�������30 s����Һ���ָ�ԭ������ɫ���ٶ���
��iii���ɿ�ͼ�����ݼ��㣬�ɵ�ʯӢ��A����������Ԫ�صİٷֺ���Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com