
ÏÂÁĐÓĐčŰË”·šŐęÈ·”ÄÊÇ
A.žÖÌú”ÄžŻÊŽčęłÌÖĐŁŹÎöÇâžŻÊŽÓëÎüŃőžŻÊŽČ»żÉÄÜÍŹÊ±·ąÉú
B.·ŽÓŠNH4Cl(s)ŁœNH3(g)+HCl(g)ÔÚÊÒÎÂÏÂČ»ÄÜŚÔ·ąœűĐĐŁŹÔòžĂ·ŽÓŠ”ÄŠ€HŁŸ0
C.ÓÉÓÚKsp(BaSO4)ŁŒKsp(BaCO3)ŁŹÒòŽËBaSO4łÁ”íČ»żÉÄÜŚȘ»ŻÎȘBaCO3 łÁ”í
D.25ĄæʱŁŹ0.1 molĄ€LŁ1CH3COOHÈÜÒșŒÓËźÏĄÊÍșóŁŹc(OHŁ)/c(CH3COOH)ÔöŽó

| Äꌶ | žßÖĐżÎłÌ | Äꌶ | łőÖĐżÎłÌ |
| žßÒ» | žßÒ»Ăâ·ŃżÎłÌÍÆŒöŁĄ | łőÒ» | łőÒ»Ăâ·ŃżÎłÌÍÆŒöŁĄ |
| žß¶ț | žß¶țĂâ·ŃżÎłÌÍÆŒöŁĄ | łő¶ț | łő¶țĂâ·ŃżÎłÌÍÆŒöŁĄ |
| žßÈę | žßÈęĂâ·ŃżÎłÌÍÆŒöŁĄ | łőÈę | łőÈęĂâ·ŃżÎłÌÍÆŒöŁĄ |
żÆÄżŁșžßÖĐ»ŻŃ§ ÀŽÔŽŁș ÌâĐÍŁș
ŸĘ±š”ÀŁŹ»ŻșÏÎïM¶Ô·ŹÇŃ»ÒĂčŸúÓĐœÏșĂ”ÄÒÖŸú»îĐÔŁŹÆäșÏłÉ·ÏßÈçÏÂÍŒËùÊŸŁș

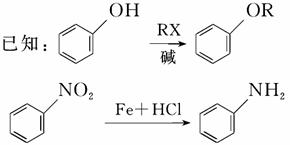
ÍêłÉÏÂÁĐÌîżŐŁș
(1)ĐŽłö·ŽÓŠÀàĐÍŁș
·ŽÓŠąÛ________Ł»·ŽÓŠąÜ________ĄŁ
(2)ĐŽłöœáččŒòÊœŁș
A_____________________________________________________________Ł»
E__________________________________________________________ĄŁ
(3)ĐŽłö·ŽÓŠąÚ”Ä»ŻŃ§·œłÌÊœŁș_______________________________________
_________________________________________________________________ĄŁ
(4)B”ÄșŹ±œ»·œáčč”ÄÍŹ·ÖÒìččÌćÖĐŁŹÓĐÒ»ÀàÄÜ·ąÉúŒîĐÔËźœâŁŹĐŽłöŒìŃéŐâÀàÍŹ·ÖÒìččÌćÖĐ”ÄčÙÄÜÍĆ(·ÓôÇ»ùłęÍâ)”ÄÊÔŒÁŒ°łöÏÖ”ÄÏÖÏóŁș
ÊÔŒÁ(·ÓÌȘłęÍâ)Łș___________________________________________Ł»
ÏÖÏóŁș_____________________________________________________ĄŁ
(5)ĐŽłöÁœÖÖC”ÄșŹ±œ»·œáččÇÒÖ»șŹ4ÖÖȻ͏»ŻŃ§»·ŸłÇâÔŚÓ”ÄÍŹ·ÖÒìččÌć”ÄœáččŒòÊœŁș
_________________________________________________________
_________________________________________________________ĄŁ
(6)·ŽÓŠąÙĄą·ŽÓŠąÚ”ÄÏÈșóŽÎĐòČ»Äܔߔ裏ÇëœâÊÍÔÒòŁș_________________
____________________________________________________________ĄŁ
Č鿎Žđ°žșÍœâÎö>>
żÆÄżŁșžßÖĐ»ŻŃ§ ÀŽÔŽŁș ÌâĐÍŁș
ÓĂaĄąbÁœžöÖÊÁżÏà”È”ÄPt”猫”çœâAlCl3șÍCuSO4”Ä»ìșÏÈÜÒș[nŁšAlCl3Ł©ŁșnŁšCuSO4Ł©=1Łș9]ĄŁt1ʱżÌa”猫”Ă”œ»ìșÏÆűÌ棏ÆäÖĐCl2ÔÚ±êŚŒŚŽżöÏÂÎȘ224 mLŁšșöÂÔÆűÌć”ÄÈÜœâŁ©Ł»t2ʱżÌCuÈ«ČżÔڔ猫ÉÏÎöłöĄŁÏÂÁĐĆжÏŐęÈ·”ÄÊÇ Łš Ł©
AŁźa”猫Óë”çÔŽ”ÄžșŒ«ÏàÁŹ
BŁźt2ʱŁŹÁœ”猫”ÄÖÊÁżÏàČî3Łź84 g
CŁź”çœâčęłÌÖĐŁŹÈÜÒș”ÄpHČ»¶ÏÔöŽó
DŁźt2ʱŁŹb”Ĕ猫·ŽÓŠÊÇ4OH-Ò»4e-=2H2O+O2Ąü
Č鿎Žđ°žșÍœâÎö>>
żÆÄżŁșžßÖĐ»ŻŃ§ ÀŽÔŽŁș ÌâĐÍŁș
ÏÂÁĐĐđÊöŐęÈ·”ÄÊÇ Łš Ł©
AŁźœ«ÏĄ°±ËźÖđ”ÎŒÓÈëÏĄÁòËáÖĐŁŹ”±ÈÜÒșpH=7ʱŁŹcŁšSO42ŁŁ©ŁŸcŁšNH4Ł«Ł©
BŁźÁœÖÖŽŚËáÈÜÒș”ÄÎïÖÊ”ÄÁżĆš¶È·Ö±đÎȘc1șÍc2ŁŹpH·Ö±đÎȘașÍa+1ŁŹÔòc1=10c2
CŁźpH=11”ÄNaOHÈÜÒșÓëpH=3”ÄŽŚËáÈÜÒș”ÈÌć»ę»ìșÏŁŹ”ÎÈëÊŻÈïÈÜÒșłÊșìÉ«
DŁźÏò0Łź1 mol/L”Ä°±ËźÖĐŒÓÈëÉÙÁżÁòËáï§čÌÌ棏ÔòÈÜÒșÖĐ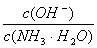 ÔöŽó
ÔöŽó
Č鿎Žđ°žșÍœâÎö>>
żÆÄżŁșžßÖĐ»ŻŃ§ ÀŽÔŽŁș ÌâĐÍŁș
ÏÂÁĐÓĐčŰÊ”ŃéŐęÈ·”ÄÊÇ
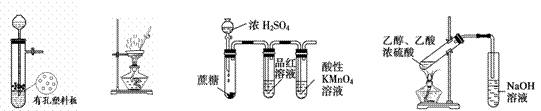 |
͌1 ͌2 ͌3 ͌4
A.ÍŒ1Ś°ÖĂÓĂÓÚCușÍĆšH2SO4·ŽÓŠÖÆÈĄÉÙÁż”ÄSO2ÆűÌć
B.ÍŒ2Ś°ÖĂÓĂÓÚŚÆÉŐAl(OH)3
C.ÍŒ3Ś°ÖĂÓĂÓÚŒìŃéĆšÁòËáÓëŐáÌÇ·ŽÓŠČúÉú”ĶțŃő»ŻÁò
D.ÍŒ4Ś°ÖĂÓÉÓÚÊ”ŃéÊÒÖƱžÒÒËáÒÒő„
Č鿎Žđ°žșÍœâÎö>>
żÆÄżŁșžßÖĐ»ŻŃ§ ÀŽÔŽŁș ÌâĐÍŁș
Éú»îÖĐÏÂÁĐÎïÖÊ”ÄÓŠÓĂ»òÏÖÏóœâÊÍŽíÎó”ÄÊÇŁš Ł©
AŁźÊłŃοɌś”śÎ¶ŒÁŁŹÒȿɌśÊłÆ··ÀžŻŒÁ
BŁźŽóÁżÈŒÉŐ»ŻÊŻÈŒÁÏÊÇÔìłÉÎíöČÌìÆű”ÄÖŰÒȘÒòËŰÖźÒ»
CŁźŚ°ÊÎČÄÁÏÊÍ·Ć”ÄŒŚÈ©»áÔìłÉżŐÆűÎÛÈŸ
DŁźÄ„¶čœŹ”ÄŽó¶čž»șŹ”°°ŚÖÊŁŹ¶čœŹÖó·Đșó”°°ŚÖʱäłÉÁË°±»ùËá
Č鿎Žđ°žșÍœâÎö>>
żÆÄżŁșžßÖĐ»ŻŃ§ ÀŽÔŽŁș ÌâĐÍŁș
ÏÂÁĐĐđÊöÖĐŁŹŐęÈ·”ÄÊÇŁš Ł©
AŁźÊŻÓÍĄąĂșĄąÌìÈ»ÆűĄąÇâÆű¶ŒÊôÓÚ»ŻÊŻÈŒÁÏ
BŁźłŁÎÂÏÂŁŹ·ŽÓŠCŁšsŁ©Ł«CO2ŁšgŁ©Łœ2COŁšgŁ©Č»ÄÜŚÔ·ąœűĐĐŁŹÔòžĂ·ŽÓŠ”ÄŠ€H<0
CŁźÈËĂÇÍšłŁÓĂ±êŚŒÈŒÉŐÈÈ»òÈÈÖ”ÀŽșâÁżÈŒÁÏÈŒÉŐ·ĆłöÈÈÁż”ÄŽóĐĄŁŹÄłÎïÖÊ”ÄÈÈÖ”ÔœžßÔòÆä±êŚŒÈŒÉŐÈÈÔœŽó
DŁźÁœžöÌć»ęÏàÍŹ”ÄÈĘÆśÖĐłäÈë”ÈÁż”ÄNO2·ąÉú·ŽÓŠŁș2NO2ŁšgŁ©
 N2O4ŁšgŁ© Š€HŁŒ0ŁŹŸűÈÈÈĘÆśÖĐÆűÌć”ÄŃŐÉ«±ÈșăÎÂÈĘÆśÖĐŃŐÉ«Éî
N2O4ŁšgŁ© Š€HŁŒ0ŁŹŸűÈÈÈĘÆśÖĐÆűÌć”ÄŃŐÉ«±ÈșăÎÂÈĘÆśÖĐŃŐÉ«Éî
Č鿎Žđ°žșÍœâÎö>>
żÆÄżŁșžßÖĐ»ŻŃ§ ÀŽÔŽŁș ÌâĐÍŁș
ŚÔ2009Äê1ÔÂ1ÈŐżȘÊŒŁŹÎÒčúÆđŐśÈŒÓÍË°ĄŁŒőÉÙÆûÓÍ”ÈłÉÆ·ÓÍ”ÄÊčÓĂÊÇŐâŽÎË°·ŃžÄžï”ÄÄż”ÄÖźÒ»ĄŁÏÂÁĐË”·šČ»ŐęÈ·”ÄÊÇ(ĄĄĄĄ)
AŁźč€Ò”ÉÏœ«ÊŻÓÍŐôÁó”Ă”œÆûÓÍŁŹ·ąÉúÁË»ŻŃ§±ä»Ż
BŁźÆûł”ÎČÆűÖĐ”ÄÌŒÇ⻯șÏÎï»áŒÓŸçÎÂÊÒЧӊ
CŁźÆûł”ÎČÆű”ÄŽóÁżĆĆ·ĆÊÇĐÎłÉč⻯ѧŃÌÎí”ÄÖŰÒȘÔÒò
DŁźÆûł”ÎČÆűÖДĥ°șÚŃÌĄ±»áÔöŒÓżŐÆűÖĐčÌÌćżĆÁŁ”ÄșŹÁż
Č鿎Žđ°žșÍœâÎö>>
żÆÄżŁșžßÖĐ»ŻŃ§ ÀŽÔŽŁș ÌâĐÍŁș
ÔÚ2 LÈĘ»ęČ»±ä”ÄÈĘÆśÖĐŁŹ·ąÉúN2Ł«3H2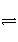
 2NH3”Ä·ŽÓŠĄŁÏÖÍšÈë4 mol H2șÍ4 mol N2,10 sÄÚÓĂH2±íÊŸ”Ä·ŽÓŠËÙÂÊÎȘ0.12 mol/(L·s)ŁŹÔò10 sșóÈĘÆśÖĐN2”ÄÎïÖÊ”ÄÁżÊÇ(ĄĄĄĄ)
2NH3”Ä·ŽÓŠĄŁÏÖÍšÈë4 mol H2șÍ4 mol N2,10 sÄÚÓĂH2±íÊŸ”Ä·ŽÓŠËÙÂÊÎȘ0.12 mol/(L·s)ŁŹÔò10 sșóÈĘÆśÖĐN2”ÄÎïÖÊ”ÄÁżÊÇ(ĄĄĄĄ)
AŁź1.6 mol BŁź2.8 mol CŁź3.2 mol DŁź3.6 mol
Č鿎Žđ°žșÍœâÎö>>
°Ù¶ÈÖÂĐĆ - Á·Ï°ČáÁбí - ÊÔÌâÁбí
șț±±ÊĄ»„ÁȘÍű΄·šșÍČ»ÁŒĐĆÏąŸÙ±šÆœÌš | ÍűÉÏÓĐșŠĐĆÏąŸÙ±šŚšÇű | ”çĐĆŐ©ÆŸÙ±šŚšÇű | ÉæÀúÊ·ĐéÎȚÖśÒćÓĐșŠĐĆÏąŸÙ±šŚšÇű | ÉæÆóÇÖÈšŸÙ±šŚšÇű
΄·šșÍČ»ÁŒĐĆÏąŸÙ±š”ç»°Łș027-86699610 ŸÙ±šÓÊÏäŁș58377363@163.com