��2013?������һģ����þ�Ͻ����ִ����졢������������ҵ����Ҫ���ϣ�Ϊ�ⶨij��þ�Ͻ𣨲�������Ԫ�أ���þ������������ijʵ��С�����������ʵ�鷽����ÿ����������ȡ5.4g��ĩ״��Ʒ����̽�����밴Ҫ��ش��������⣺
[ʵ��l]��þ�Ͻ�
�ⶨʣ�����������
��֪���Լ�X��ɫ��ӦΪ��ɫ��
��1���������õ���Ʒ����V
1mL 2.0mol/L�Լ�X�У���ַ�Ӧ���˷�Ӧ�����ӷ���ʽΪ
2Al+2OH-+2H2O�T2AlO2-+3H2��
2Al+2OH-+2H2O�T2AlO2-+3H2��
��V
1��ȡֵ��Χ��
��100mL
��100mL
mL��
��2�������ˡ�����������壬���þ����������
ƫ��
ƫ��
���ƫ�ߡ�����Ӱ�족��ƫ�͡�������ԭ����
���˺�û��ϴ��
���˺�û��ϴ��
��
[ʵ��2]��þ�Ͻ�
| ����O2������� |
|
| �ܷ�(�����������ƶ��Ļ���) |
�ⶨ����
��l��������þ��������������ʵ�黹��ⶨ��������
���պ���������
���պ���������
��
��2�����ÿ�������O
2����ʵ�飬��ⶨþ������������
ƫ��
ƫ��
�ƫ�ߡ�����Ӱ�족��ƫ�͡�����[��֪��3Mg+N
2Mg
3N
2]
[ʵ��3]��þ�Ͻ�
�ⶨ������ɫ��������
ʵ��װ������ͼ��
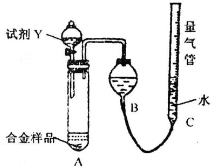
��1����ʵ����ʢװҩƷ֮ǰӦ���Ƚ��е�ʵ�鲽����
���װ�õ�������
���װ�õ�������
��
��2��ͬѧ��ѡ���Լ�Y����ʵ�飬������װ��A����ƷѸ���ܽ���ȫ����������������C��Һ����B��Һ����ƽ����ò����������ΪV
2L[������Ϊ��״���£�A��Һ��������Բ��ƣ�������3����ͬ]���Լ�Y��������
ϡ�����ϡ����
ϡ�����ϡ����
������þ����������ƫ�͵�������
���ȷ�Ӧ���¶����ߣ��ⶨ�����������ƫ����Al�ĺ���Խ����������Խ�࣬�������������ʱ�����Mg����������ƫ��
���ȷ�Ӧ���¶����ߣ��ⶨ�����������ƫ����Al�ĺ���Խ����������Խ�࣬�������������ʱ�����Mg����������ƫ��
��
��3��ͬѧ�ҽ��Լ�Y��Ϊʵ��1�е��Լ�X�������������ȷ����ַ�Ӧ���ò����������ΪV
3L����þ����������Ϊ
����V
3�ı���ʽ����






