
ij��ȤС����Ƴ�ͼ��ʾװ�����Ľ��̲��С�ͭ�����ᷴӦ��ʵ�飬��̽����ѧʵ�����ɫ����
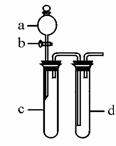
(�̶�װ����ȥ)
(1)ʵ��ǰ������b���Թ�d�м�ˮ����û�����ܿڣ������Թ�c��d�Ľ���������c����Ŀ���� ��
(2)��d�м�����NaOH��Һ��c�з�һС��ͭƬ���ɷ�Һ©��a��c�м���2 mLŨ���ᣬc�з�Ӧ�Ļ�ѧ����ʽ�� ������a��c�м�2 mL����ˮ��c�е�ʵ�������� ��
(3)��27������ȡ����ͭ�����ַ�������������ɫ��ѧ�������ѷ����� ��������______________ ��
(4)��С�黹������װ�ý���ʵ��֤��������KMnO4��Cl2��Br2����������Ϊ ��ʵ������Ϊ ������ʵ��IJ���֮���� ��
���𰸡�
(1)���װ�������ԡ�
(2)Cu+4HNO3(Ũ)=Cu(NO3)2+2NO2��+2H2O
��Ӧ�仺��������ɫ�䵭��
(3)�������������٣�����Ⱦ��
(4)��d�м���KBr��Һ��c�м������KMnO4����a��c�м���Ũ���c���л���ɫ���������d����Һ��Ϊ����ɫ��û�д���β����
����������������ɫ��ѧΪ���壬������˼ά��ȫ���Ժ���ѡ������
���ַ������£��ף�Cu+4HNO3(Ũ)===Cu(NO3)2+2NO2��+2H2O��
�ң�3Cu+8HNO3(ϡ)===3Cu(NO3)2+2NO��+4H2O��
��:2Cu+O2 2CuO��
2CuO��
CuO+2HNO3===Cu(NO3)2+H2O��
���ַ�����ȣ����������������٣�û����Ⱦ����������������˻������ܵ�ԭ��
(4)�е����ԭ��Ϊ��16HCl+2KMnO4===2KCl+2MnCl2+5Cl2��+8H2O�٣�Cl2+2Br��===Br2+2Cl���ڡ�
���â��в�����Cl2��������Ӧ�ڣ����������
��d�м���KBr��Һ��c�м������KMnO4����a��c�м���ŨHCl��c�в����Ļ���ɫ�������d����������Br2��Һ�����ɫ����û��β������װ�á�


 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ�������Ԥ��ʵ��Ŀ�Ļ�����ʵ�����һ�µ���
| ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� |
| A | ij�����������ᣬ������ʹ����ʯ��ˮ����ǵ���ɫ��ζ���� | ˵���ü����� |
| B | �������� | ��ȥ |
| C | �����£��� | ˵��������
|
| D |
| �����Ƶ������Ƿ�Ϊ��ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ע�⣺����Ϊ�ֲ��⣬��A��B���⣬��������ѡһ�⡣�����������һ�ɰ�A��Ʒ֡�A���ʺ�ʹ�ö��ڿθ��½̲ĵĿ������B���ʺ�ʹ��һ�ڿθĽ̲ĵĿ������
��A������ͼ��ʾ�����ס�������װ�в�ͬ���ʵ���Ͳ�õ�������������������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ�ͬʵ�飨������ͬ��ͬѹ�²ⶨ����

| ʵ����� | ����Ͳ������ | ����Ͳ������ | ����Ͳ������ |
| 1 | 10 mL FeSO4��Һ | 10 mL NH3 | ���ɰ�ɫ���������ɫ |
| 2 | 20 mL H2S | 10 mL SO2 | |
| 3 | 30 mL NO2����Ҫ�� | 10 mL H2O(l) | ʣ����ɫ���壬�����Զ�����ѹ�� |
| 4 | 15 mL Cl2 | 40 mL NH3 |
�Իش��������⣺
��1��ʵ��1�У��������ձ�Ϊ___________ɫ��д��������ɫ�Ļ�ѧ����ʽ_______________��
��2��ʵ��2����Ͳ�ڵ������ǣ���________���ɣ�����___________�ƶ�������⡱�����ڡ�����������Ӧ�����Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��__________��Һ�С�
��3��ʵ��3�У����е�30 mL������NO2��N2O4�Ļ�����壬��ô�������ʣ�����ɫ������__________��д��NO2��H2O��Ӧ�Ļ�ѧ����ʽ_______________________________��
��4��ʵ��4�У���֪��3Cl2+2NH3 N2+6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�仯Ϊ___________�������Ͳ��ʣ����������ԼΪ______________mL��
N2+6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�仯Ϊ___________�������Ͳ��ʣ����������ԼΪ______________mL��
��B��ijʵ��С��������װ�ý����Ҵ���������ʵ�顣

��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ
_____________________________________________________________________
_____________________________________________________________________��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�������Ӧ��________��Ӧ��
��2����������ˮԡ���ò���ͬ��
��������____________________���ҵ�������_____________________��
��3����Ӧ����һ��ʱ������Թ�a�����ռ�����ͬ�����ʣ�������____________________________������ƿ���ռ������������Ҫ�ɷ���______________��
��4�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����__________��Ҫ��ȥ�����ʣ������ڻ��Һ�м���______________����д��ĸ����
a.�Ȼ�����Һ  b.��
b.��
c.̼��������Һ d.���Ȼ�̼
Ȼ����ͨ��_____________����ʵ��������ƣ����ɳ�ȥ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���ܴﵽԤ��Ŀ�ĵ���
| ��� | ʵ������ | ʵ��Ŀ�� |
| A | ��SO2ͨ������KMnO4��Һ�� | ֤��SO2���������� |
| B | ��Cl2ͨ��NaBr��Һ�� | �Ƚ��������������ǿ�� |
| C | ��ͭ��Ũ���ᷴӦ���ɵ������ռ����ñ�ˮ�������ȴ���� | �о��¶ȶԻ�ѧƽ���Ӱ�� |
| D | �ֱ���2֧�Թ��м�����ͬ�����ͬŨ�ȵ�H2O2��Һ����������1֧��������MnO2 | �о�������H2O2�ֽ����ʵ�Ӱ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ӽ���ij��÷��������֣�
| ���鷽�� | ������ | ��ɫ�� | ���巨 |
| ���� | ��Ӧ���г����������ܽ� | ��Ӧ������ɫ�仯 | ��Ӧ����������� |
�������Ӽ���ķ�������������
A NH4+�����巨 B I����������
C Fe3+����ɫ�� D Ca2+�����巨
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ºʹ���ѹǿ�£���ͼʾ��װ�ý���ʵ�飬���ag��CaC290%����Ʒ��ˮ��ȫ��Ӧ�������������bL����������ͬ�����£��ⶨij��ʯ������CaC2��������������ش��������⣺

��1��CaC2��ˮ��Ӧ�Ļ�ѧ����ʽ��___________________________��
��2������Ӧ�ս���ʱ���۲쵽��ʵ��������ͼ��ʾ����ʱ��������ȡ�������ܣ�������___________________________��
��3����ʵ���в����������ʱӦע���������___________________________________��
��4�������ʯ��������Ϊcg������������ΪdL�����ʯ������CaC2��������������ʽw��CaC2����_______________________�������������ɵ����� ������Բ��ƣ���
������Բ��ƣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͭ�ж�����;��������������ɫ��������������ȡ�Ϊ��ô���������ͭ��̽�������ʣ�ijͬѧ�ù�ҵ����ͭ(����������������)��������ʵ�飺
���Ʊ�����ͭ
��ҵCuSO4
 CuSO4��Һ
CuSO4��Һ CuSO4��5H2O��������CuO
CuSO4��5H2O��������CuO
�ٲ���I��Ŀ���dz����������ʡ������� ��
�ڲ�����Ŀ���dz����������ǣ��μ�H2O2��Һ���Լ��ȣ���Fe2+ת����ȫ����������Cu2(OH)2CO3��ĩ�����裬�Կ�����ҺpH=3.5���������һ��ʱ�䣬���ˣ���ϡ�����ữ��Һ��pH=1��������ҺpH=3.5��ԭ���� ��
�۲�����Ŀ���ǵõ�CuSO4��5H2O���塣������ �����ˣ�ˮԡ���Ⱥ�ɡ�ˮԡ���ȵ��ص��� ��
��̽������ͭ������
��ȡA��B��֧�Թܣ���A���ȼ�������CuO��ĩ���ٷֱ���A��B�м���������3% H2O2��Һ��ֻ�۲쵽A���д������ݡ������� ��
��Ϊ̽���Թ�A�з�Ӧ�����ʣ��ռ����岢�ⶨ����������ʵ�������У�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ȵ������£�ֻ��һ���Լ��Ϳ��Լ���(NH4)2SO4��KCl��MgCl2��Al2(SO4)3��Fe2(SO4)3��Һ�������Լ��� (����)
A��NaOH B��NH3��H2O
C��AgNO3 D��BaCl2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ�У�E��F�ֱ�Ϊ��Դ���������ձ���ʢ��100 mL 0.2mol��L��1 AgNO3��Һ�����ձ���ʢ��100 mL 0.15 mol��L��1 CuCl2��Һ��A��B��C��D��Ϊʯī�缫��������һ��ʱ�����B������1.08g����
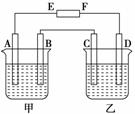
(1)EΪ________����CΪ________����
(2)A���ĵ缫��ӦʽΪ_________________________����������________mL��(��״��)
(3)�����ձ���Һ������䣬���ʱ��Һ�����ʵ���Ũ�Ƚ���Ϊ mol��L��1��
(4)���ձ��е���ʯ����Һ��________��������죨��A��B�������������⣬�ڼ��ձ������յõ�________��Һ(�����ʵĻ�ѧʽ)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com