
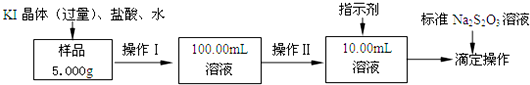
·ÖĪö £Ø1£©ĀČ»ÆĢś±„ŗĶČÜŅŗµĪČė·ŠĖ®ÖŠ¼ÓČČÉś³ÉĒāŃõ»ÆĢś½ŗĢ壻
£Ø2£©øł¾ŻÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗĖłŠčŅŖµÄŅĒĘ÷ÓŠ£ŗĮæĶ²”¢½ŗĶ·µĪ¹Ü”¢ÉÕ±”¢²£Į§°ō”¢Ņ»¶Ø¹ęøńµÄČŻĮæĘ棻
£Ø3£©ŅĄ¾ŻĮ÷³ĢÖŠČÜŅŗĢå»żµÄ¾«Č·¶ČæÉÖŖ£¬100.00mLµÄČÜŅŗŠčŅŖ¾«ĆÜĮæ¾ßĮæČ”£»µāÓöµķ·Ū±äĄ¶É«£¬Ėę±ź×¼Na2S2O3ČÜŅŗµĪČėŗĶµāµ„ÖŹ·“Ó¦£¬ČÜŅŗĄ¶É«±äĪŖĪŽÉ«ĒŅ°ė·ÖÖÓ²»ĶŹÉ«£»
£Ø4£©ŅĄ¾Ż·“Ó¦µÄ¶ØĮæ¹ŲĻµ¼ĘĖćµĆµ½£¬×¢ŅāČÜŅŗĢå»żµÄ±ä»Æ£»
£Ø5£©ŅŖ°ŃѳʷĀČ»ÆĢśÖŠµÄÉŁĮæFeCl2ŌÓÖŹ³żČ„£¬ŠčŅŖ¼ÓČėŃõ»Æ¼ĮŃõ»ÆŃĒĢśĄė×ÓĪŖĢśĄė×Ó£¬µ«¼ÓČėµÄŃõ»Æ¼Į²»ÄÜŅżČėŠĀµÄŌÓÖŹ£»
£Ø6£©ŅĄ¾ŻŹµŃé²Ł×÷¹ż³Ģ·ÖĪö³ĮµķŠčŅŖ¹żĀĖŗóĻ“µÓ³żČ„±ķĆęµÄŌÓÖŹ£¬¼ģŃéŹĒ·ń³ĮµķĶźČ«£¬æÉŅŌŌŚÉĻ²ćĒåŅŗÖŠ¼ÓČė°±Ė®¹Ū²ģŹĒ·ńÓŠ³ĮµķÉś³É£»³Įµķ³ĘĮæŗćÖŲµÄ±ź×¼ŹĒĮ½“Ī³ĘĮæÖŹĮæĻąĶ¬»ņĻą²ī²»³¬¹ż0.001g£®
½ā“š ½ā£ŗ£Ø1£©Č”ÉŁĮæĀČ»ÆĢśŃłĘ·µĪČė50mL·ŠĖ®ÖŠ£¬¼ÓČČʬæĢ£¬ŅŗĢå³ŹĻÖŗģŗÖÉ«£¬Éś³ÉµÄŹĒĒāŃõ»ÆĢś½ŗĢ壬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖFe3++3H2O?Fe£ØOH£©3+3H+£¬
¹Ź“š°øĪŖ£ŗFe3++3H2O?Fe£ØOH£©3+3H+£»
£Ø2£©ŅņÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗĖłŠčŅŖµÄŅĒĘ÷ÓŠ£ŗĮæĶ²”¢½ŗĶ·µĪ¹Ü”¢ÉÕ±”¢²£Į§°ō”¢Ņ»¶Ø¹ęøńµÄČŻĮæĘ棬
¹Ź“š°øĪŖ£ŗ100mLČŻĮæĘ棻½ŗĶ·µĪ¹Ü£»
£Ø3£©100.00mLµÄČÜŅŗŠčŅŖ¾«ĆÜĮæ¾ßĮæČ”£¬ÉÕ±ŹĒ“ÖĀŌĮæČ”£¬ĮæĶ²Ö»Äܾ«Č·µ½0.1mL£¬ĖłŅŌÓƵĪ¶Ø¹Ü¾«Č·µ½0.01mL£¬Ń”ÓƵĪ¶Ø¹ÜĮæČ”ČÜŅŗ100.00mLµÄČÜŅŗ£»µāÓöµķ·Ū±äĄ¶É«£¬Ėę±ź×¼Na2S2O3ČÜŅŗµĪČėŗĶµāµ„ÖŹ·“Ó¦£¬×īŗóŅ»µĪ±ź×¼ŅŗµĪČėŹ±£¬×¶ŠĪĘæÖŠČÜŅŗÓÉĄ¶É«±äĪŖĪŽÉ«£¬ĒŅ°ė·ÖÖÓÄŚ²»±äÉ«£¬ĖµĆ÷“ļµ½·“Ó¦ÖÕµć£»
¹Ź“š°øĪŖ£ŗd£»×īŗóŅ»µĪ±ź×¼ŅŗµĪČėŹ±£¬×¶ŠĪĘæÖŠČÜŅŗÓÉĄ¶É«±äĪŖĪŽÉ«£¬ĒŅ°ė·ÖÖÓÄŚ²»±äÉ«£»
£Ø4£©2Fe3++2I-”ś2Fe2++I2£¬I2+2S2O32-”ś2I-+S4O62-£¬2FeCl3-6H2O”«2Fe3+”«I2”«2S2O32-£»µĪ¶ØŹ±£¬10.00mlČÜŅŗÖŠµāµ„ÖŹĻūŗÄÅضČĪŖ0.1000mol/LµÄ±ź×¼Na2S2O3ČÜŅŗ18mL£¬FeCl3-6H2OµÄĪļÖŹµÄĮæ=0.1000mol/L”Į0.018L=0.0018mol£¬øĆѳʷ֊100.00mLČÜŅŗÖŠĖłŗ¬FeCl3•6H2OµÄĪļÖŹµÄĮæĪŖ0.018mol£¬ÖŹĮæ·ÖŹż=$\frac{0.018mol”Į270.5g/mol}{5.0g}$”Į100%=97.38%£»
¹Ź“š°øĪŖ£ŗ97.38%£»
£Ø5£©ŅŖ°ŃѳʷĀČ»ÆĢśÖŠµÄÉŁĮæFeCl2ŌÓÖŹ³żČ„£¬ŠčŅŖ¼ÓČėŃõ»Æ¼ĮŃõ»ÆŃĒĢśĄė×ÓĪŖĢśĄė×Ó£¬µ«¼ÓČėµÄŃõ»Æ¼Į²»ÄÜŅżČėŠĀµÄŌÓÖŹ£»
a£®Ģś·ŪŗĶĢśĄė×Ó·“Ó¦£¬²»ÄÜŗĶ ŃĒĢśĄė×Ó·“Ó¦£¬¹Źa²»·ūŗĻ£»
b£®ĀČĖ®æÉŅŌŃõ»ÆŃĒĢśĄė×ÓĪŖĢśĄė×Ó£¬ĒŅ²»ŅżČėŠĀµÄŌÓÖŹ£¬¹Źb·ūŗĻ£»
c£®äåĖ®ÄÜŃõ»ÆŃĒĢśĄė×Ó£¬µ«ŅżČėĮĖäåĄė×Ó£¬¹Źc²»·ūŗĻ£»
d£®Ė«ŃõĖ®æÉŅŌŃõ»ÆŃĒĢśĄė×ÓĪŖĢśĄė×Ó£¬¹żŃõ»ÆĒā±»»¹ŌĪŖĖ®£¬²»ŅżČėŌÓÖŹ£¬¹Źd·ūŗĻ£»
¹ŹŃ”bd£»
£Ø6£©ŹµŃé²Ł×÷¹ż³Ģ·ÖĪö³ĮµķŠčŅŖ¹żĀĖŗóĻ“µÓ³żČ„±ķĆęµÄŌÓÖŹ£¬¼ģŃéŹĒ·ń³ĮµķĶźČ«£¬æÉŅŌŌŚÉĻ²ćĒåŅŗÖŠ¼ÓČė°±Ė®¹Ū²ģŹĒ·ńÓŠ³ĮµķÉś³É£»³Įµķ³ĘĮæŗćÖŲµÄ±ź×¼ŹĒĮ½“Ī³ĘĮæÖŹĮæĻąĶ¬»ņĻą²ī²»³¬¹ż0.001g£¬
¹Ź“š°øĪŖ£ŗĻ“µÓ£»³Įµķ¾²ÖĆŌŚ²ćĒåŅŗÖŠ£¬¼ÓČė°±Ė®ČÜŅŗ¹Ū²ģÓŠĪŽ³ĮµķÉś³É£¬ČōĪŽ³ĮµķÉś³ÉÖ¤Ć÷³ĮµķĶźČ«£»Į½“Ī³ĘĮæµÄÖŹĮæĻąµČ»ņĻą²ī²»³¬¹ż0.001g£®
µćĘĄ ±¾Ģāæ¼²éĮĖĪļÖŹ×é³ÉŗĶŠŌÖŹµÄŹµŃéŃéÖ¤ŗĶŹµŃéĢ½¾æ·½·Ø£¬Ģś¼°Ęä»ÆŗĻĪļŠŌÖŹµÄ·ÖĪöÓ¦ÓĆ£¬ĪļÖŹ³żŌÓ£¬µĪ¶ØŹµŃé²ā¶ØĪļÖŹŗ¬ĮæµÄ¼ĘĖćÓ¦ÓĆÅŠ¶Ļ£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢ŚÖŠĖ®µÄµēĄė³Ģ¶ČµÄŹĒ¢ŁµÄ8±¶ | |
| B£® | ¢ŁŗĶ¢ŪÖŠĖ®µÄµēĄė³Ģ¶ČĻąµČ | |
| C£® | µČĢå»żµÄ¢ŁŗĶ¢Ü»ģŗĻŗóĖłµĆČÜŅŗÖŠ£ŗc£ØNH3•H2O£©£¼c£ØNH4+£© | |
| D£® | ¢ŚÖŠĖ®µÄµēĄė³Ģ¶ČµČÓŚ¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | m=2 | |
| B£® | Į½“ĪĘ½ŗāµÄĘ½ŗā³£ŹżĻąĶ¬£¬Ę½ŗā³£ŹżÖµĪŖ2 | |
| C£® | XÓėYµÄĘ½ŗā×Ŗ»ÆĀŹÖ®±ČĪŖ1£ŗ1 | |
| D£® | µŚ¶ž“ĪĘ½ŗāŹ±£¬ZµÄÅضČĪŖ1.0 mol•L-1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ū¢Ü | B£® | ¢Ł¢Ü¢Ž | C£® | ¢Ś¢Ū¢Ż | D£® | ¢Ū¢Ż¢Ž |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com