
��9�֣����к�NaCl��Na2SO4��NaNO3�Ļ���ѡ���ʵ����Լ�����ת��Ϊ��Ӧ�ij�������壬�Ӷ�ʵ��Cl-��SO42-����NO3-������롣��Ӧ��ʵ����̿�����ͼ��ʾ��
��ش��������⣺
��д��ʵ���������������ʵĻ�ѧʽ�� �Լ�X _______������A��_______������B��__________��
������ʵ�������м��������Na2CO3��Ŀ����____________________________
�ǰ���ʵ�鷽���õ�����Һ3�п϶�����_____________���ѧʽ�����ʣ�Ϊ�˽��������⣬��������Һ3�м���������_________��֮����Ҫ��ù���NaNO3����е�ʵ�������___________________ ����������ƣ�.
 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1������Ͳȡ��Ʒ��Һ2mL���Թ��У��ٵμ�������2mol/L���ᣬ������۲� | �� ��Һ����ɫ���ɫ ��Һ����ɫ���ɫ �������1���ܳ���������Һ�����Ա仯 ��Һ�����Ա仯 ��������2���ܳ��� ����2���ܳ��� �� |
| ����2��������1 ���Թ��м��� 1mL�� 1mL�� ��������۲� |
����Һ�ֲ㣬�ϲ��л�����ֳ�ɫ��Ⱥ�ɫ�������2������ ����Һֻ�ֲ㣬����ɫ�仯�������1���� ����Һ�ֲ㣬�ϲ��л�����ֳ�ɫ��Ⱥ�ɫ�������2������ ����Һֻ�ֲ㣬����ɫ�仯�������1���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| m |
| M |
| m |
| M |
| 2mNA |
| M |
| 2mNA |
| M |
| 22.4m |
| M |
| 22.4m |
| M |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
�����Ǿ�������С�ظ���λ�����ڿռ䲻����չ���ɾ��塣NaCl������һ���������壨��ͼ�������ǰ����������ӿ��ɲ��Ⱦ���Բ���˴����У����Ӽ��ļ����������������ӵİ뾶֮�ͣ���ͼ������֪aΪ������������������⣺
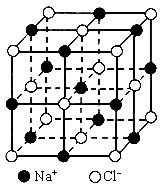
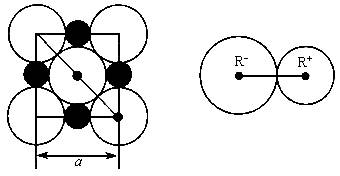
��1��ÿ��������ƽ����̯________��Na+��________��Cl-��
��2����ijNaCl���������Ϊ5.85g����Լ��________mol NaCl������
��3��NaCl�������Ӽ��ļ���Ϊ________��Na+���Ӱ뾶��Cl-���Ӱ뾶֮��Ϊ![]() ________��
________��
��4��NaCl���岻���ڷ��ӣ����ڸ����£����ڵ���1413��ʱ������ת�������NaCl�ķ�����ʽ���ڣ�����1mol NaCl���壬��ǿ��ʹ������������������Ϊ11.2L������Ϊ��״���������ʱ�Ȼ�������ķ���ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
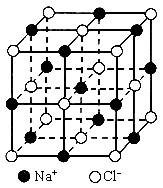
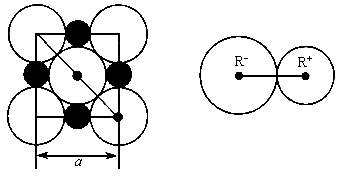
��1��ÿ��������ƽ����̯________��Na+��________��Cl-��
��2����ijNaCl���������Ϊ5.85g����Լ��________mol NaCl������
��3��NaCl�������Ӽ��ļ���Ϊ________��Na+���Ӱ뾶��Cl-���Ӱ뾶֮��Ϊ![]() ________��
________��
��4��NaCl���岻���ڷ��ӣ����ڸ����£����ڵ���1413��ʱ������ת�������NaCl�ķ�����ʽ���ڣ�����1mol NaCl���壬��ǿ��ʹ������������������Ϊ11.2L������Ϊ��״���������ʱ�Ȼ�������ķ���ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��֪ʶ����������ѵ������������ѧ ���ͣ�022
�����ᄃ������С�ظ���λ�����ڿռ䲻����չ���ɾ��壮NaCl������һ����������(ͼ��������)�����ǰ����������ӿ��ɲ��Ⱦ���Բ���˴����У����Ӽ��ļ����������������ӵİ뾶֮��(��ͼ)����֪aΪ������������������⣺
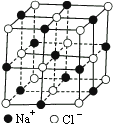
(1)ÿ��������ƽ����̯________��Na+��________��Cl����
(2)��ijNaCl���������Ϊ5.85�ˣ���Լ��________Ħ��NaCl������
(3)NaCl�������Ӽ��ļ���Ϊ________��Na+���Ӱ뾶��Cl�����Ӱ뾶֮��Ϊ![]() ��________��
��________��
(4)NaCl���岻���ڷ��ӣ����ڸ�����(��1413��ʱ)����ת�������NaCl�ķ�����ʽ���ڣ�����1mol NaCl���壬��ǿ��ʹ������������������Ϊ11.2��(����Ϊ���)�����ʱ�Ȼ�������ķ���ʽΪ________��
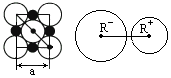
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com