
| A��CH4(g)��2NO2(g)===N2(g)��CO2(g)��2H2O(l)����H����867 kJ��mol��1 |
| B��CH4����ԭNOxΪN2�Ĺ����У���x��1.6����ת�Ƶ���3.2 mol |
| C����0.2 mol CH4��ԭNO2��N2�������������·ų�������Ϊ173.4 kJ |
| D�����ñ�״����4.48 L CH4��ԭNO2��N2������������ת�Ƶ���3.2 mol |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 O2(g)
O2(g) CO(g) ��H1 = ��110.5 kJ/mol
CO(g) ��H1 = ��110.5 kJ/mol O2(g)
O2(g) CO2(g)����H2 = ��283.0 kJ/mol
CO2(g)����H2 = ��283.0 kJ/mol CO2(g)�ķ�Ӧ��Ϊ(����)
CO2(g)�ķ�Ӧ��Ϊ(����)| A��172.5 kJ/mol | B����172.5 kJ/mol | C��393.5 kJ/mol | D����393.5 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���ڢ� | C���ڢ� | D���٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2Q2=Q1 | B��2Q2<Q1 | C��Q2<Q1<197kJ | D��Q2=Q1<197kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��2A + B | B��2C |
| C��2A(g) + B(g) | D��2A(g) + B(g) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ȳ��ȼ����Ϊ1256kJ/mol |
| B����ת��10mol���ӣ�������2.5mol O2 |
| C��������2molҺ̬ˮ�����H����2512kJ/mol |
| D�����γ�4mol̼�����õ��Ӷԣ���ų�������Ϊ2512kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH4(g)��2NO2(g) ��N2(g)��CO2(g)��2H2O(g)��H����867kJ��mol-1 |
| B��CH4(g)��4NO2(g) ��4NO(g)��CO2(g)��2H2O(l)��H3����H1 |
| C������0.2mol CH4��ԭNO2��N2����Ӧ�зų�������һ��Ϊ173.4kJ |
| D�����ñ�״����2.24L CH4��ԭNO2��N2������������ת�Ƶĵ���Ϊ1.6mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ȼ����Ϊ-890.3kJ��mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ�� CH4(g)+2O2(g)=CO2(g)+2H2O(g)��H= -890.3kJ��mol-1 |
B��500�桢30MPa�£���0.5mol N2��1.5molH2�����ܱյ������г�ַ�Ӧ����NH3(g)������19.3kJ�����Ȼ�ѧ����ʽΪ��N2(g)+3H2(g) 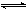 2NH3(g)��H= -38.6kJ��mol-1 2NH3(g)��H= -38.6kJ��mol-1 |
| C��1 g H2��ȫȼ������Һ̬ˮ�ų�142.9 kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ�� H2��g����1/2O2��g��="=" H2O( l ) ��H����142.9 kJ��mol��1 |
| D����֪25�桢101kPa�£�ʯī�����ʯȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com