
| A | B | C | D | |
| Na2O2��NaHCO3�����ʵ���֮�� | 8��1 | 9��2 | 1��8 | 2��9 |
| ԭϡ��������ʵ���Ũ�ȣ�mol/L�� | 3.4 | 1.1 | 1.8 | 1.3 |
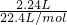 =0.1mol��
=0.1mol��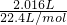 =0.09mol��
=0.09mol��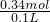 =3.4mol/L��
=3.4mol/L��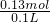 =1.3mol/L��
=1.3mol/L��

 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ������Na2O2��NaHCO3�Ļ�Ϸ�ĩ��Ϊ���ȷݣ�������һ�ݼ���100mLϡ������ǡ����ȫ��Ӧ�����ɵ������������Ϊ2.24L����״���£����ٽ�������ͨ����һ�ݻ�����У���ַ�Ӧ�õ�O2 2.016L����״���£�����ԭ��Ϸ�ĩ��Na2O2��NaHCO3�����ʵ���֮�ȼ�ϡ��������ʵ���Ũ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2012?�Ϻ�ģ�⣩��һ������Na2O2��NaHCO3��Ϸ�ĩ��Ϊ���ȷݣ�������һ�ݼ��뵽100mLϡ������ǡ����ȫ��Ӧ�����ɸ��������2.24L����״�������ٽ�������ͨ�뵽��һ�ݻ�����У���ַ�Ӧ���������Ϊ2.016L����״��������ԭ��Ϸ�ĩ��Na2O2��NaHCO3�����ʵ���֮�ȼ�ԭϡ��������ʵ���Ũ�ȿ����ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��3.4mol?L-1 | B��0.2mol?L-1 | C��1.8mol?L-1 | D��3.6 mol?L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ������Na2O2��NaHCO3��Ϸ�ĩ��Ϊ���ȷݣ�������һ�ݼ��뵽100mLϡ������ǡ����ȫ��Ӧ�����ɸ��������2��24L����״�������ٽ�������ͨ�뵽��һ�ݻ�����У���ַ�Ӧ���������Ϊ2��016L����״��������ԭ��Ϸ�ĩ��Na2O2��NaHCO3�����ʵ���֮�ȼ�ԭϡ��������ʵ���Ũ�ȿ����ǡ��������������������� �� ��
|
| A | B | C | D |
| Na2O2��NaHCO3�����ʵ���֮�� | 8:1 | 9:2 | 1:8 | 2:9 |
| ԭϡ��������ʵ���Ũ�ȣ�mol/L�� | 3��4 | 1��1 | 1��8 | 1��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���Ϻ���ʮУ�����ڶ���������ѧ�Ծ� ���ͣ���ѡ��
��һ������Na2O2��NaHCO3��Ϸ�ĩ��Ϊ���ȷݣ�������һ�ݼ��뵽100mLϡ������ǡ����ȫ��Ӧ�����ɸ��������2��24L����״�������ٽ�������ͨ�뵽��һ�ݻ�����У���ַ�Ӧ���������Ϊ2��016L����״��������ԭ��Ϸ�ĩ��Na2O2��NaHCO3�����ʵ���֮�ȼ�ԭϡ��������ʵ���Ũ�ȿ����ǣ� ��
| | A | B | C | D |
| Na2O2��NaHCO3�����ʵ���֮�� | 8:1 | 9:2 | 1:8 | 2:9 |
| ԭϡ��������ʵ���Ũ�ȣ�mol/L�� | 3��4 | 1��1 | 1��8 | 1��3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com