
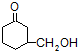
 ���Ǻϳ�������ҩ����Ҫԭ�ϣ����ĺϳ�·�����£�
���Ǻϳ�������ҩ����Ҫԭ�ϣ����ĺϳ�·�����£�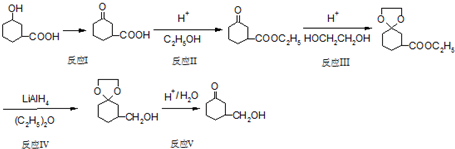
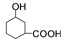 �к��еĹ���������
�к��еĹ��������� +CH3CH2OH
+CH3CH2OH| H+ |
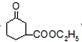 +H2O
+H2O +CH3CH2OH
+CH3CH2OH| H+ |
 +H2O
+H2O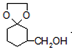 +
+| H+ |
 +HOCH2CH2OH
+HOCH2CH2OH +
+| H+ |
 +HOCH2CH2OH
+HOCH2CH2OH ���Ҵ�����������Ӧ��
���Ҵ�����������Ӧ�� �Ľṹ��֪�����еĹ�����Ϊ-OH��-COOH��
�Ľṹ��֪�����еĹ�����Ϊ-OH��-COOH�� ���Ҵ�����������Ӧ��
���Ҵ�����������Ӧ�� ����ˮ�⡢��ȥ1����ˮ����
����ˮ�⡢��ȥ1����ˮ���� �������ɶ�Ԫ����
�������ɶ�Ԫ���� ���Ҵ�����������Ӧ��
���Ҵ�����������Ӧ�� �Ľṹ��֪�����еĹ�����Ϊ-OH��-COOH������Ϊ�ǻ����Ȼ���
�Ľṹ��֪�����еĹ�����Ϊ-OH��-COOH������Ϊ�ǻ����Ȼ��� ���Ҵ�����������Ӧ����Ӧ����ʽΪ��
���Ҵ�����������Ӧ����Ӧ����ʽΪ�� +CH3CH2OH
+CH3CH2OH| H+ |
 +H2O��
+H2O�� +CH3CH2OH
+CH3CH2OH| H+ |
 +H2O��
+H2O�� ����ˮ�⡢��ȥ1����ˮ����
����ˮ�⡢��ȥ1����ˮ���� �������ɶ�Ԫ������Ӧ����ʽΪ��
�������ɶ�Ԫ������Ӧ����ʽΪ�� +
+| H+ |
 +HOCH2CH2OH��
+HOCH2CH2OH�� +
+| H+ |
 +HOCH2CH2OH��
+HOCH2CH2OH��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ������һ�и�����һ��������⻯ѧ�Ծ����������� ���ͣ�������
�����dz���������ǿ��֮һ���ڻ�ѧ�о��ͻ������������Ź㷺Ӧ�ã��������Ʊ������Ρ�Ⱦ�ϡ����ϡ�ҽҩ�м��塢����ըҩ�ȡ������ζ���������Լ���ͼ������ҵ��
��1��ij����M������������ʱ����ʽ�ֽ⣺2MNO3 2M+2NO2��+O2��������3.40gMNO3������NO2��O2����ɱ�״��ʱ�������Ϊ672mL���ɴ˿��Լ����M�����ԭ������Ϊ_____________��
2M+2NO2��+O2��������3.40gMNO3������NO2��O2����ɱ�״��ʱ�������Ϊ672mL���ɴ˿��Լ����M�����ԭ������Ϊ_____________��
��2����32.64gͭ��140mL һ��Ũ�ȵ����ᷴӦ��ͭ��ȫ�ܽ������NO��NO2�����������ɱ�״���µ����Ϊ11.2L������NO�����Ϊ_____________��
��3������Cu��Cu2O��CuO��ɵĻ���ij�о���ѧϰС��Ϊ��̽����������������100mL0.6mol/LHNO3��Һǡ��ʹ�������ȫ�ܽ⣬ͬʱ�ռ���224mLNO���壨��״�����������������ͭ�����ʵ���Ϊ ����ԭ���������0.0lmolCu��������Cu2O��CuO��������Ϊ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�����и�����һ��������⻯ѧ�Ծ��������棩 ���ͣ�������
�����dz���������ǿ��֮һ���ڻ�ѧ�о��ͻ������������Ź㷺Ӧ�ã��������Ʊ������Ρ�Ⱦ�ϡ����ϡ�ҽҩ�м��塢����ըҩ�ȡ������ζ���������Լ���ͼ������ҵ��
��1��ij����M������������ʱ����ʽ�ֽ⣺2MNO3 2M+2NO2��+O2��������3.40gMNO3������NO2��O2����ɱ�״��ʱ�������Ϊ672mL���ɴ˿��Լ����M�����ԭ������Ϊ_____________��
2M+2NO2��+O2��������3.40gMNO3������NO2��O2����ɱ�״��ʱ�������Ϊ672mL���ɴ˿��Լ����M�����ԭ������Ϊ_____________��
��2����32.64gͭ��140mL һ��Ũ�ȵ����ᷴӦ��ͭ��ȫ�ܽ������NO��NO2�����������ɱ�״���µ����Ϊ11.2L������NO�����Ϊ_____________��
��3������Cu��Cu2O��CuO��ɵĻ���ij�о���ѧϰС��Ϊ��̽����������������100mL0.6mol/LHNO3��Һǡ��ʹ�������ȫ�ܽ⣬ͬʱ�ռ���224mLNO���壨��״�����������������ͭ�����ʵ���Ϊ ����ԭ���������0.0lmolCu��������Cu2O��CuO��������Ϊ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ������
 )�Ǻϳ�������ҩ����Ҫԭ�ϣ����ĺϳ�·������
)�Ǻϳ�������ҩ����Ҫԭ�ϣ����ĺϳ�·������
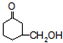
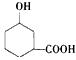 �ж���ͬ���칹�壬���к���
�ж���ͬ���칹�壬���к��� ��ͬ���칹����__________��д��������5�����ʵĽṹ��ʽ����
��ͬ���칹����__________��д��������5�����ʵĽṹ��ʽ�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com