
����Ŀ��CoS2��CO����������й����Ĺ�ҵ��ǰ�����ش��������⣺
(1)��֪��
CoS2(s) +CO(g) ![]() CoS(s) +COS(g) H1
CoS(s) +COS(g) H1
2COS(g) +SO2(g) ![]() 3S(s) +2CO2(g) H2
3S(s) +2CO2(g) H2
S(s) +CoS(S) ![]() CoS2 (s) ��H3
CoS2 (s) ��H3
��2CO(g)+ SO2(g)![]() 2CO2(g)+S(s) H4=____�� (��H1�� H2��H3��ʾ)
2CO2(g)+S(s) H4=____�� (��H1�� H2��H3��ʾ)
(2)�ں��¡���ѹ��������ģ���������SO2��ʼ����Ϊ1mol�����CO2��ƽ�����������CO��SO2��Ͷ�ϱȱ仯��ͼ��

�ٵ�Ͷ�ϱ�Ϊ2ʱ��t min ʱ���SO2ת����Ϊ50%������S���������ʱ�ʾ�ķ�Ӧ����v=______g��min-1��
�ڵ�Ͷ�ϱ�Ϊ3ʱ��CO2 ��ƽ�����������Ӧ�ĵ���______________��
(3)�������Ϊ1L�ĺ��¡������������ͨ��2 mol CO��| mol SO2����Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��
�������I��II�ı�����������____________________��
��SO2��ƽ��ת����Ϊ______��ƽ�ⳣ��Kp =________(��ƽ���ѹ����ƽ��Ũ�ȼ���)��
(4)���õ�ⷨ����SO2β�����Ʊ����շ� (Na2S2O4).���װ����ͼ����a____ b (����>�� ��=������<��)������S2O42-�ĵ缫��ӦʽΪ____________________��

���𰸡�![]()
![]() C ʹ�ã���ʹ�ø���Ч������ 75% 0.675 <
C ʹ�ã���ʹ�ø���Ч������ 75% 0.675 < ![]()
��������
��1�����ݸ�˹���ɿ�֪![]() �ɵ������Ȼ�ѧ����ʽ��
�ɵ������Ȼ�ѧ����ʽ��
��2�����������η�����S����������S����������![]() ���㣻�ڵ�Ͷ�ϱ�Ϊ3ʱ���൱����Ͷ�ϱ�Ϊ2�ﵽƽ��ʱ����1mol��CO��ƽ�����ƣ�������������ԭ����֪�ﵽƽ��ʱ��CO2���������С��Ͷ�ϱ�Ϊ2�ﵽƽ��ʱCO2�����������
���㣻�ڵ�Ͷ�ϱ�Ϊ3ʱ���൱����Ͷ�ϱ�Ϊ2�ﵽƽ��ʱ����1mol��CO��ƽ�����ƣ�������������ԭ����֪�ﵽƽ��ʱ��CO2���������С��Ͷ�ϱ�Ϊ2�ﵽƽ��ʱCO2�����������
��3������ͼ���֪I��II���ﵽƽ��ʱѹǿ���䣬��ƽ�ⲻ�ƶ���II�ﵽƽ��ʱ���̣���IIʹ�ã���ʹ�ø���Ч���������ں��¡�������������������ѹǿ�����ʵ��������ȣ��������η�����SO2��ƽ��ת���ʣ�ƽ�ⳣ��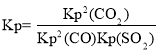 ���Դ˼��㣻
���Դ˼��㣻
��4���ɵ��װ��ͼ��֪��SO2����������Ӧ����H2SO4��Ϊ�������缫��ӦΪ��![]() ��HSO3-������ԭ��Ӧ����S2O42-��Ϊ�������缫��ӦΪ��
��HSO3-������ԭ��Ӧ����S2O42-��Ϊ�������缫��ӦΪ��![]() ���Դ˷�����
���Դ˷�����
��1��![]()
![]()
![]() ���ݸ�˹���ɿ�֪
���ݸ�˹���ɿ�֪![]() �ɵ�
�ɵ�![]()
![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��2���ٵ�Ͷ�ϱ�Ϊ2ʱ��t min ʱ���SO2ת����Ϊ50%������
S����������![]() ���ڵ�Ͷ�ϱ�Ϊ3ʱ���൱����Ͷ�ϱ�Ϊ2�ﵽƽ��ʱ����1mol��CO��ƽ�����ƣ�������������ԭ����֪�ﵽƽ��ʱ��CO2���������С��Ͷ�ϱ�Ϊ2�ﵽƽ��ʱCO2������������ʴ�Ϊ��
���ڵ�Ͷ�ϱ�Ϊ3ʱ���൱����Ͷ�ϱ�Ϊ2�ﵽƽ��ʱ����1mol��CO��ƽ�����ƣ�������������ԭ����֪�ﵽƽ��ʱ��CO2���������С��Ͷ�ϱ�Ϊ2�ﵽƽ��ʱCO2������������ʴ�Ϊ��![]() ��C��
��C��
��3������ͼ���֪I��II���ﵽƽ��ʱѹǿ���䣬��ƽ�ⲻ�ƶ���II�ﵽƽ��ʱ���̣���IIʹ�ã���ʹ�ø���Ч���������ں��¡�������������������ѹǿ�����ʵ��������ȣ��跴Ӧ����SO2���ʵ���Ϊxmol������
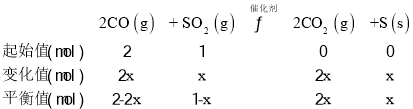
![]() ��x=0.75mol��SO2��ƽ��ת����Ϊ
��x=0.75mol��SO2��ƽ��ת����Ϊ![]() ����ƽ��ʱn��CO��=0.5mol��n��SO2��=0.25mol��n��CO2��=1.5mol����ƽ�ⳣ��
����ƽ��ʱn��CO��=0.5mol��n��SO2��=0.25mol��n��CO2��=1.5mol����ƽ�ⳣ��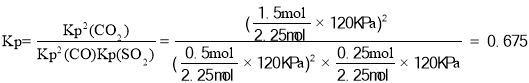 ���ʴ�Ϊ��ʹ�ã���ʹ�ø���Ч��������75%��0.675��
���ʴ�Ϊ��ʹ�ã���ʹ�ø���Ч��������75%��0.675��
��4���ɵ��װ��ͼ��֪��SO2����������Ӧ����H2SO4��Ϊ�������缫��ӦΪ��![]() ��������Ũ������HSO3-������ԭ��Ӧ����S2O42-��Ϊ�������缫��ӦΪ��
��������Ũ������HSO3-������ԭ��Ӧ����S2O42-��Ϊ�������缫��ӦΪ��![]() ���ʴ�Ϊ��<��
���ʴ�Ϊ��<��![]() ��
��


 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���й�������ĸ���������мƻ����в�����ǰ�ƽ�����һ�ҹ�����ĸĿǰ���ڽ��к��ԡ����캽ĸ��Ҫ���������Ͳ��ϡ���ĸ������Ҫ�ͳ������ĸ�ļװ�Ҫ���£���ĸ�����Ҫ��ʴ��
(1)�����ֿ���ʴ����ǿ��Ni2+��̬ԭ�ӵĺ�������Ų�Ϊ_______����Ԫ�������ڱ���______����
(2)��ĸ�װ�Ϳ��һ�����µIJ��Ͼ۹�����ṹ��ͼ��ʾ������Cԭ���ӻ���ʽΪ_______�ӻ���
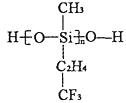
(3)������Ԫ�ص�ҡ������ˮ�к��д���±��Ԫ�ء�
�ٸ����±������жϣ����п������ɽ��ȶ��ĵ��������ӵ�±��ԭ����______��Ԫ�ط���
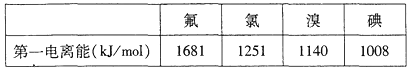
�ڸ��ݼ۲���ӶԻ������ۣ�Ԥ��ClO3���Ŀռ乹��Ϊ______�Σ�д��һ��ClO3���ĵȵ�����Ļ�ѧ����______��
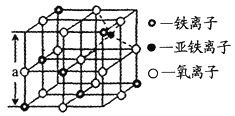
(4)�������������������ŵ�һ�����ɫ������̲��Ŵ�������Դ�����й衢�����̡�п�ȡ�����ͼ�Ǵ����������Ӿ���Fe3O4��ȡ�����������侧��ṹ��һ�������壬�����е������Ƿ��������������ܶѻ���_____(����������������)�����������Dz���Fe3O4�ľ�����______(����������������)���������������Ӵ���������Χ�ɵ�_______(��ռ�ṹ)��϶��������ͼ����Fe3O4������ܶ�Ϊ________g/cm3��(ͼ��a=0.42nm��������������λ��Ч����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ������˵���������
A. 11.2 L �������ϩ�Ļ�����к���ԭ����Ŀ����2NA
B. ��NA�� CO32���� Na2CO3��Һ�У�Na+��Ŀ����2NA
C. �ܱ������У�2 molSO2������ O2��ַ�Ӧ������ķ�����С��2NA
D. 4.0 g CO2�����к�������Ŀ����2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������⻯��ͭ(CuH)����CuSO4��Һ�͡���һ�ַ�Ӧ���40��~50��ʱ��Ӧ����.CuH���ȶ����ֽ⣻CuH����������ȼ�գ������¸����ᷴӦ�ܲ������壬�����й������ƶ��в���ȷ����()
A.����һ�ַ�Ӧ�һ������������B.CuH�ȿ���������Ҳ������ԭ��
C.2CuH + 3Cl2![]() 2CuCl2 + 2HCl��D.CuH+HCl=CuCl��+H2��
2CuCl2 + 2HCl��D.CuH+HCl=CuCl��+H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��DCCNa (��������������)������һ�ָ�Ч����ȫ����������20������������ˮ��(CNO)3H3 (������)Ϊ��Ԫ���ᡣ
I.�Ʊ�DCCA (������������)װ����ͼ����Ҫ��Ӧ�У�
���� (CNO)3H3+ 2NaOH=(CNO)3Na2H + 2H2O ��H<0
�Ȼ� (CNO)3Na2H +2Cl2=(CNO)3Cl2H + 2NaCl H<0
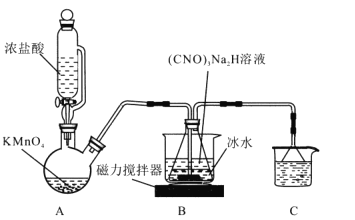
�ش��������⣺
(1)װ��C�е���Һ��______________������Ϊ______________��
(2)װ��A�з�Ӧ�����ӷ���ʽΪ_______________��
(3)װ��B�ñ�ˮԡ��ԭ����__________�� ����ʱ���������ƹ�����(CNO)3Na2H�п��ܻ��е�������__________��
��.�Ʊ�DCCNa
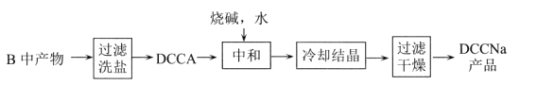
(4)����II�й���ϴ����ϴȥ������__________________��
(5)���к�����Ӧ�Ļ�ѧ����ʽΪ_________________��
(6)��Ԫ�غ����IJⶨ����ȡ0. 1000 g DCNa��Ʒ������һ����������Һ�ܽ⣬��Ʒ�е���Ԫ��ȫ��ת����HClO���ټ���������KI��Һ���õ�����ָʾ���� ��01000 mol��L-1Na2S2O3����Һ�ζ����ɵĵ⣬����VmL.��֪�� I2+2S2O32-=2I-+S4O62-����Ʒ����Ԫ�ص���������=__________%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��Ԥ��21������Ҷ�����������ܾ�����ʱ�����������ʶ��Ǿ��й���Ӧ��ǰ���Ĵ�����ϡ��ش��������⣺
(1)Zr(�)��Ԫ�����ڱ���λ�ڵ������ڣ�����ͬ�壬��̬Zr�ļ۲�����Ų�ʽΪ_______��
(2)�ǰ����(Li2NH) ����Ԫ�ص�һ��������С����____ ���縺��������_____ (��Ԫ�ط���)��
(3)����(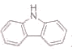 )�ķе����(
)�ķе����(![]() )�ߵ���Ҫԭ����________��
)�ߵ���Ҫԭ����________��
(4)��NH3BH3 (�����飬�۵�104��)�����黥Ϊ�ȵ����塣NH3BH3�ľ�������Ϊ____������B���ӻ�����Ϊ____����ͨ��_________�ⶨ�÷��ӵ����幹�͡�
��NH3BH3��ͨ�������顢CH4��H2O���кϳɣ����ǣ� CH4______H2O (����> "����<")��ԭ����________��
(5)MgH2�������ķ�Ʒϵ���ṹ��ͼ����������a =b= 450pm�� c= 30lpm��ԭ������ΪA(0��0��0)��B(0.305��0.305��0)��C(1��1��1)��D(0.195��0.805��0.5)��
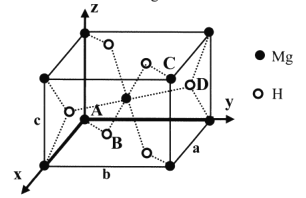
��Mg2+�İ뾶Ϊ72pm����H+�İ뾶Ϊ______pm (�г��������ʽ)
����NA��ʾ�����ӵ�������MgH2����������ܶ��DZ�״���������ܶȵ�_____��(�г��������ʽ�������ܶ�Ϊ0.089g��L-1)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʱ�������ݻ���ͬ����ƿ�зֱ�ʢ�������������壨ͬ��ͬѹ�������ɼ�K��ʹ����ƿ�ڵ������ֻ�Ϻ������ڵ�ѹǿ��С����
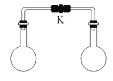
A.H2S�� SO2B.NH3��HCl
C.H2��Cl2D.NO�� O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��![]() �Ⱦ����ҹ�����������������Թ�ѧ���塣
�Ⱦ����ҹ�����������������Թ�ѧ���塣
��1��![]() ����Ԫ�صĵ縺���ɴ�С��˳����__________________��
����Ԫ�صĵ縺���ɴ�С��˳����__________________��![]() �ĵ�һ�����ܴӴ�С��˳����______________________��
�ĵ�һ�����ܴӴ�С��˳����______________________��
��2����̬��ԭ�ӵĺ�������Ų�ʽΪ____________________��![]() ���۵��
���۵��![]() �ĵͣ���ԭ����___________________________________________��
�ĵͣ���ԭ����___________________________________________��
��3��![]() ����25%��
����25%��![]() ��Һ�У��ڲ��ƻ�Ǧ��������Ũ���ɵ��ķ������
��Һ�У��ڲ��ƻ�Ǧ��������Ũ���ɵ��ķ������![]() ���壬����
���壬����![]() �����м�����450��ɵ�
�����м�����450��ɵ�![]() ��
��
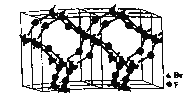
����̬![]() ���Ե�����
���Ե�����![]() ���ڣ���̬
���ڣ���̬![]() �Ľṹ��ͼ��ʾ����̬
�Ľṹ��ͼ��ʾ����̬![]() ���̬
���̬![]() ������ԭ���ӻ���ʽ����Ϊ_____________��_________________��
������ԭ���ӻ���ʽ����Ϊ_____________��_________________��
��![]() �Ŀռ乹��Ϊ________________������
�Ŀռ乹��Ϊ________________������![]() �Ľṹ:_______________________.
�Ľṹ:_______________________.
��4���Ծ�������Ϊ��λ���Ƚ���������ϵ���Ա�ʾ�����и�ԭ�ӵ�λ�ã�����ԭ�ӷ������꣬![]() �����ṹ��ͼ��ʾ��Ʒ�������
�����ṹ��ͼ��ʾ��Ʒ�������![]() ������Զ��
������Զ��![]() ��ԭ�ӷ�������Ϊ_______________��
��ԭ�ӷ�������Ϊ_______________��![]() ��Ħ������Ϊ
��Ħ������Ϊ![]() ���������ܶ�Ϊ
���������ܶ�Ϊ![]() ��������
��������![]() ____________pm���ô���ʽ��ʾ��
____________pm���ô���ʽ��ʾ��
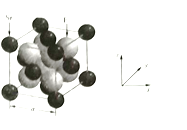
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A.  ��
��![]() ������ͬ�Ĺ����ţ���Ϊͬϵ��
������ͬ�Ĺ����ţ���Ϊͬϵ��
B. 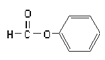 ����ȩ�࣬������Ϊ��CHO
����ȩ�࣬������Ϊ��CHO
C. ![]() ������Ϊ��2���һ���1����ϩ
������Ϊ��2���һ���1����ϩ
D. ![]() ������Ϊ��2������1��3������ϩ
������Ϊ��2������1��3������ϩ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com