
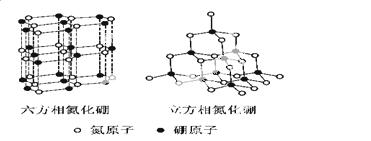
������(BN)�����ж�����ṹ�������൪������ͨ�����ڵ��ȶ��࣬��ʯī���ƣ����в�״�ṹ�������������������൪�����dz�Ӳ���ϣ����������ĥ�ԡ����ǵľ���ṹ����ͼ��ʾ��
�Ż�̬��ԭ�ӵĵ����Ų�ʽΪ ��
�� ���������־����˵������ȷ���� (�����)��
a.�����൪�����ЦҼ��ͦм�������Ӳ�ȴ� b.�����൪������������С�������ʵ���
c.���־����е�B��N����Ϊ���ۼ� d.���־����Ϊ���Ӿ���
�������൪���������һ����ԭ�������ڵ�ԭ�ӹ��ɵĿռ乹��Ϊ ����ṹ��ʯī����ȴ�����磬ԭ���� ��
�������൪�������У���ԭ�ӵ��ӻ��������Ϊ ���þ������Ȼ��������ظ�ԭ����Լ300Km�Ĺŵؿ��б����֡�������һ�����γ���ʵ���ƶ�ʵ�����������൪����ϳ������൪������Ҫ������Ӧ�� ��
��NH4BF4(�������)�Ǻϳɵ��������ܵ�ԭ��֮һ��1mo NH4BF4���� mol��λ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�ѧ������ȷ����
A��Sԭ�ӵĽṹʾ��ͼ�� B���Ȼ��Ƶĵ���ʽ��
B���Ȼ��Ƶĵ���ʽ��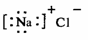
C������Ļ�ѧʽ��Na2CO3 D��̼���Ƶĵ��뷽��ʽ��NaHCO3��Na+ + H++CO32-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�������ȷ����
A������п�̵���У�MnO2 �Ǵ���
�Ǵ���
B����пŦ�۵�ع���ʱ��Ag2O����ԭΪAg
C���ŵ�ʱ��Ǧ������������Ũ�Ȳ�������
D�����ʱ�����ƵĽ�����Ʒ���淢����ԭ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڹ�ҵ�� չ��ȼ���豸�������࣬�豸��ģ����������Щ�����ŷŵ������ж����д�����
չ��ȼ���豸�������࣬�豸��ģ����������Щ�����ŷŵ������ж����д����� SO2��������ͳ�ƣ��ҹ�1995�깤ҵSO2���ŷ���Ϊ1 396��֣�2006�깤ҵSO2���ŷ����ﵽ��3 800��֣�����SO2����Ⱦ���ҹ�ÿ����ʧ�ߴ�1 100��Ԫ��
SO2��������ͳ�ƣ��ҹ�1995�깤ҵSO2���ŷ���Ϊ1 396��֣�2006�깤ҵSO2���ŷ����ﵽ��3 800��֣�����SO2����Ⱦ���ҹ�ÿ����ʧ�ߴ�1 100��Ԫ��
(1)д��������ҵ���������в���SO2��ʵ����
��________________________________________________________________________��
��________________________________________________________________________��
(2)����SO2��Ⱦ�ɲ��õĴ�ʩ��(д������)��
��________________________________________________________________________��
��________________________________________________________________________��
��___________________ _____________________________________________________��
_____________________________________________________��
(3)ʪʽʯ��ʯ—ʯ�෨��������������������������һ�ַ������乤�������ǣ���������¯Ԥ�������������������������ַ�ú���̳����پ���һ��ר�ŵ��Ƚ�������Ȼ������������������е�SO2�뺬��ʯ��ʯ�Ľ�Һ������ Һ�Ӵ���ͨ�����������ʯ��(CaSO4·2H2O)���������������Ӧ��ѭ����������������ټ��ȣ������̴ѣ����������
Һ�Ӵ���ͨ�����������ʯ��(CaSO4·2H2O)���������������Ӧ��ѭ����������������ټ��ȣ������̴ѣ����������
��д��ʪ��ʯ��ʯ—ʯ�෨�������漰�Ļ�ѧ��Ӧ����ʽ��
________________________________________________________________________
________________________________________________________________________��
����ʯ��ʯ��Һ��SO2���ռ���������ʯ������SO2��ԭ���ǣ�________________________________________________________________________
_____________ ___________________________________________________________��
___________________________________________________________��
�����������еõ���ʯ�࣬������Ȼ�����(��Ҫ��Դ��ȼ��ú)�������ʼ���ֵ����ʯ���Ʒ���ܱ仵����ҵ�������������Ȼ���ķ�����__________________��
(4)ij��ѧ��ȤС��Ϊ�˲ⶨ������������ʯ������(CaSO4·xH2O)���ⶨxֵ��������ʵ�飺��ʯ�����ʹ֮��ˮ�����ȹ����й����������ʱ��ı仯��ϵ����ͼ��ʾ�����ݱ��������������Ϊ2.72 g���ٸı䡣��
��ʯ��Ļ�ѧʽ����ͼ����AB�ζ�Ӧ������Ļ�ѧʽ��
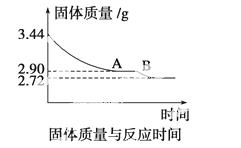
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ƶ�±����(NaX)���±����(SiX4)������������ȷ����
A��SiX4��ˮ��  B��SiX4�ǹ��ۻ�����
B��SiX4�ǹ��ۻ�����
C��NaX��ˮ�� D��NaX���۵�һ�����SiX4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��Ԫ�����ڱ���ǰ36��Ԫ��,���ǵĺ˵������������Aԭ��L��ijɶԵ�������δ�ɶԵ��������,Bԭ�ӵ������p����ĵ���Ϊ������ṹ,C�ǵؿ��к�������Ԫ�ء�D�ǵ�������Ԫ��,��ԭ�Ӻ�����������������ԭ����ͬ,���������Ӿ���������ش���������:
(1)A��B��C�ĵ�һ��������С�����˳������������(�ö�Ӧ��Ԫ�ط��ű�ʾ);��̬Dԭ�ӵĵ����Ų�ʽΪ������������������
(2)A������������Ӧ��ˮ���������,������ԭ�Ӳ�ȡ���������ӻ�;B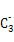 �Ŀռ乹��Ϊ��������(����������)��
�Ŀռ乹��Ϊ��������(����������)��
(3)1 mol AB-�к��еĦм�����Ϊ����������
(4)��ͼ�ǽ���Ca��D���γɵ�ij�ֺϽ�ľ����ṹʾ��ͼ,��úϽ���Ca��D��ԭ�Ӹ�����������������
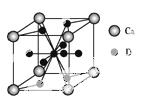
(5)�����Ͻ��������Ͻ�����ͬ���͵ľ����ṹXYn,�����к�ǿ�Ĵ�����������֪�����Ͻ�LaNin�������Ϊ9.0��10- 23 cm3,������γ�LaNinH4.5�Ͻ�(����뾧����϶,�������),��LaNin��n=��������(����ֵ);���ںϽ��е��ܶ�Ϊ����������
23 cm3,������γ�LaNinH4.5�Ͻ�(����뾧����϶,�������),��LaNin��n=��������(����ֵ);���ںϽ��е��ܶ�Ϊ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ֶ�����Ԫ�ص����ʡ���;��ԭ�ӽṹ��Ϣ���±���
| Ԫ�� | Ԫ�ص����ʡ���;��ԭ�ӽṹ��Ϣ |
| Q | ԭ�Ӻ�����6������ |
| R | �����������Ǵ�����������3�� |
| X | ��̬�⻯���ˮ��Һ���������ϣ��������� |
| Y | ��������Ԫ�صļ������������Ӱ뾶��С |
| Z | ����Ϊ����ɫ���壬�ڿ�����ȼ�շ�����ɫ���� |
����ݱ�����Ϣ�ش��������⣺
(1)Q�����̬�⻯����ӵĿռ乹��Ϊ________��
(2)R������X�������������·�Ӧ����Ϊ________������(����ۡ������ӡ�)���侧������Ϊ________��
(3)д��R��Z��ɽ������Ӽ��Ļ�����Ļ�ѧʽ��______________����ɵ���һ����������ѧ������Ϊ__________________��
(4)��ҵ���õ��Y��Z�γɻ������ˮ��Һ��ȡY���ʣ�д���÷�Ӧ�����ӷ���ʽ��_______________________��
(5)��1.01��105Pa��298 Kʱ��1.4 g QR������1.6 g R2��������ȫȼ�գ�����QR2����ʱ�ų�14.15 kJ������д��QRȼ�յ��Ȼ�ѧ����ʽ��__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ȡ��ͬ�����KI��Na2S��FeBr2��Һ���ֱ�ͨ��������Cl2������Ӧǡ�����ʱ������Cl2�������ͬ��ͬ�¡�ͬѹ�����£�����KI��Na2S��FeBr2��Һ�����ʵ���Ũ��֮���ǣ� ��
A��1��1��2 B��2��1��3 C��6��3��2 D��3��2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ�������ֵ������˵����ȷ����
A��25��ʱ��pH��13�İ�ˮ�к���OH������ĿΪ0.1NA
B��1 mol Na����ȫ��������Na2O2��ת�Ƶ��ӵ���ĿΪNA
C����״���£�2.24 LNO2 ��ˮ��Ӧ����NO3������ĿΪ0.1NA
D��4.0 g H2������������ȫ��Ӧ����NH3����Ӧ�ж��ѹ��ۼ�������Ϊ2NA
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com